Abstract
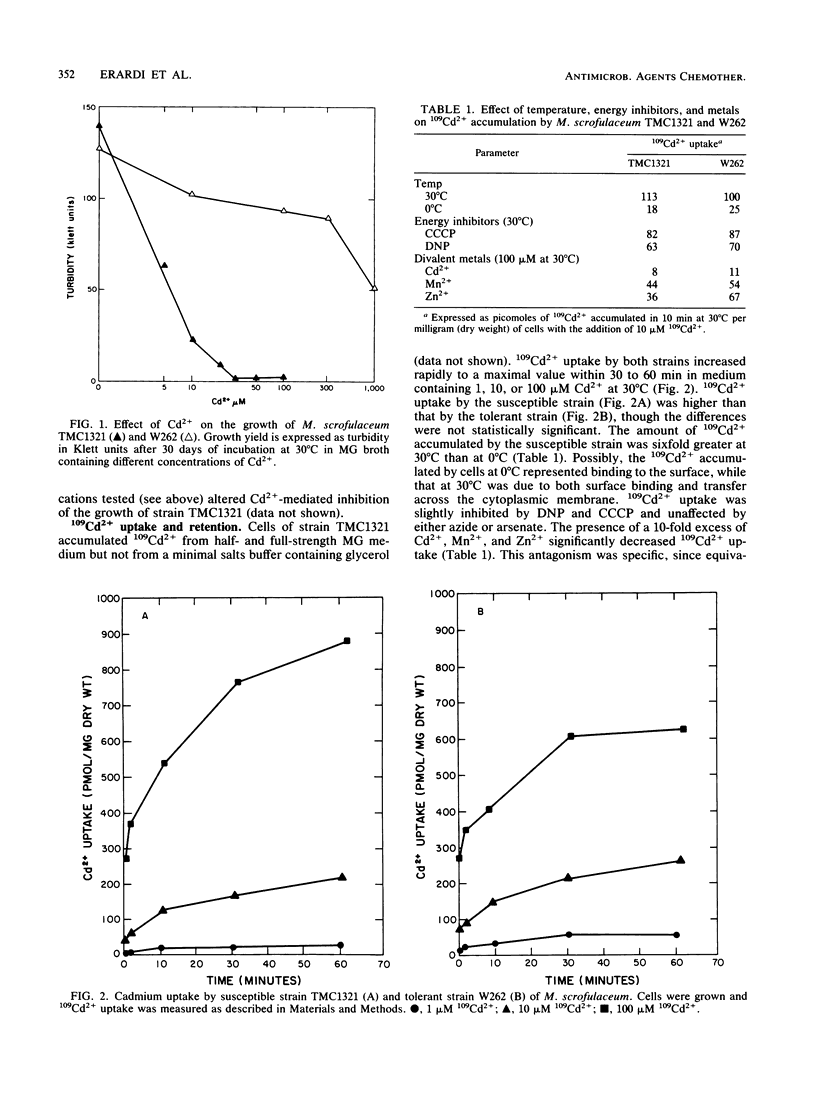
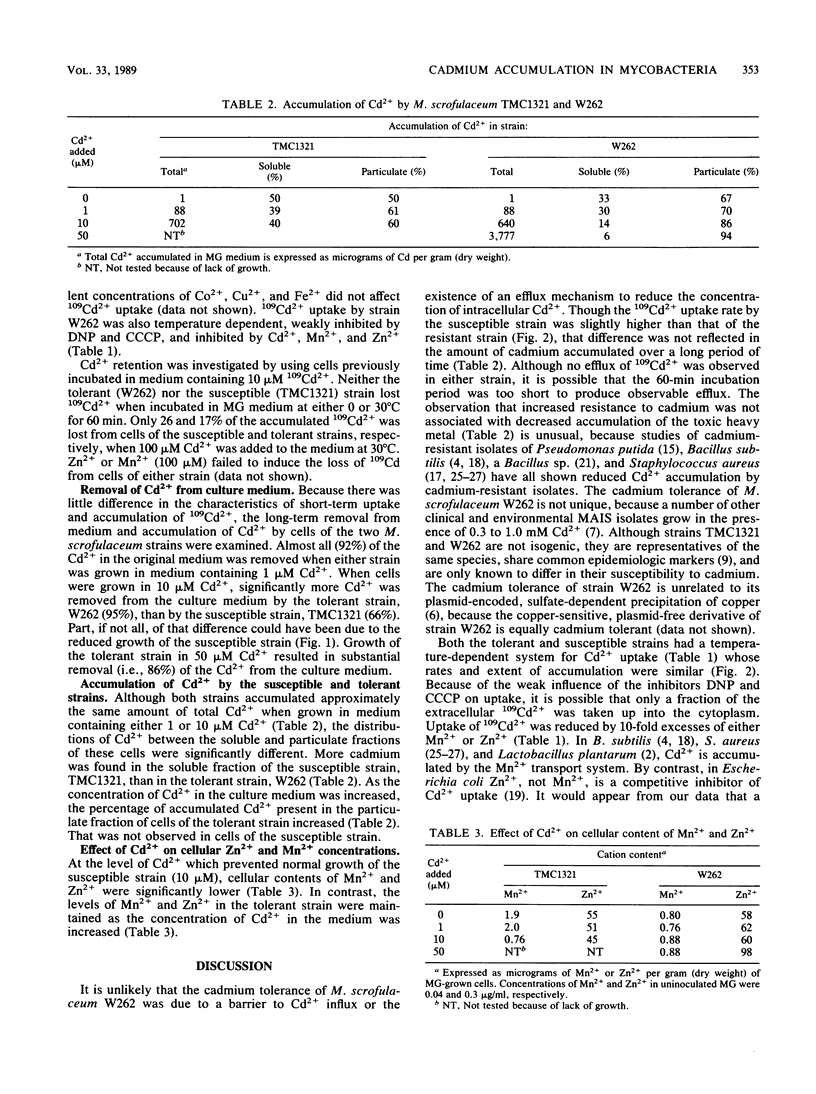
Cadmium accumulation and transport were studied in two strains of Mycobacterium scrofulaceum differing in their susceptibility to Cd2+ toxicity. A 10-fold excess of either Zn2+ or Mn2+ partially antagonized inhibition of growth by Cd2+. 109Cd2+ uptake by both the tolerant and susceptible strains was temperature dependent and inhibited by a 10-fold excess of either Zn2+ or Mn2+. There were no significant differences in either the kinetics of 109Cd2+ uptake or the retention of accumulated 109Cd2+ by the tolerant and susceptible strains. Both tolerant and susceptible strains removed most of the cadmium from the culture medium, but significantly more was removed by cells of the tolerant strain. Most of the accumulated Cd2+ in the tolerant strain was in the particulate fraction, rather than in the soluble fraction. Intracellular accumulated Cd2+ was primarily in the soluble fraction of the susceptible strain. Increased Cd2+ in culture medium resulted in decreased Mn2+ and Zn2+ in cells of the susceptible strain but did not reduce the Mn2+ and Zn2+ content of cells of the tolerant strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiking H., Stijnman A., van Garderen C., van Heerikhuizen H., van 't Riet J. Inorganic phosphate accumulation and cadmium detoxification in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl Environ Microbiol. 1984 Feb;47(2):374–377. doi: 10.1128/aem.47.2.374-377.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984 Apr;158(1):1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. W., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. V. Numbers in eastern United States soils and correlation with soil characteristics. Am Rev Respir Dis. 1984 Oct;130(4):630–633. doi: 10.1164/arrd.1984.130.4.630. [DOI] [PubMed] [Google Scholar]
- Burke B. E., Pfister R. M. Cadmium transport by a Cd2+-sensitive and a Cd2+-resistant strain of Bacillus subtilis. Can J Microbiol. 1986 Jul;32(7):539–542. doi: 10.1139/m86-100. [DOI] [PubMed] [Google Scholar]
- DARTER R. W., MILLMAN I. Localization of mycobacterial enzymes. Proc Soc Exp Biol Med. 1957 Jul;95(3):440–446. doi: 10.3181/00379727-95-23245. [DOI] [PubMed] [Google Scholar]
- Erardi F. X., Failla M. L., Falkinham J. O., 3rd Plasmid-encoded copper resistance and precipitation by Mycobacterium scrofulaceum. Appl Environ Microbiol. 1987 Aug;53(8):1951–1954. doi: 10.1128/aem.53.8.1951-1954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, George K. L., Parker B. C., Gruft H. In vitro susceptibility of human and environmental isolates of Mycobacterium avium, M. intracellulare, and M. scrofulaceum to heavy-metal salts and oxyanions. Antimicrob Agents Chemother. 1984 Jan;25(1):137–139. doi: 10.1128/aac.25.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham J. O., 3rd, Parker B. C., Gruft H. Epidemiology of infection by nontuberculous mycobacteria. I. Geographic distribution in the eastern United States. Am Rev Respir Dis. 1980 Jun;121(6):931–937. doi: 10.1164/arrd.1980.121.6.931. [DOI] [PubMed] [Google Scholar]
- Fry K. L., Meissner P. S., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. VI. Identification and use of epidemiologic markers for studies of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Am Rev Respir Dis. 1986 Jul;134(1):39–43. doi: 10.1164/arrd.1986.134.1.39. [DOI] [PubMed] [Google Scholar]
- George K. L., Falkinham J. O., 3rd Selective medium for the isolation and enumeration of Mycobacterium avium-intracellulare and M. scrofulaceum. Can J Microbiol. 1986 Jan;32(1):10–14. doi: 10.1139/m86-003. [DOI] [PubMed] [Google Scholar]
- George K. L., Parker B. C., Gruft H., Falkinham J. O., 3rd Epidemiology of infection by nontuberculous mycobacteria. II. Growth and survival in natural waters. Am Rev Respir Dis. 1980 Jul;122(1):89–94. doi: 10.1164/arrd.1980.122.1.89. [DOI] [PubMed] [Google Scholar]
- Higham D. P., Sadler P. J., Scawen M. D. Cadmium-Resistant Pseudomonas putida Synthesizes Novel Cadmium Proteins. Science. 1984 Sep 7;225(4666):1043–1046. doi: 10.1126/science.225.4666.1043. [DOI] [PubMed] [Google Scholar]
- Horitsu H., Yamamoto K., Wachi S., Kawai K., Fukuchi A. Plasmid-determined cadmium resistance in Pseudomonas putida GAM-1 isolated from soil. J Bacteriol. 1986 Jan;165(1):334–335. doi: 10.1128/jb.165.1.334-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkeala H. The distribution of cadmium between cellular subfractions in cadmium-sensitive and cadmium-resistant Staphyloccus aureus. Acta Vet Scand. 1979;20(3):438–446. doi: 10.1186/BF03546605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga R. A., Bessen R., Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985 Jun;162(3):1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laddaga R. A., Silver S. Cadmium uptake in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1100–1105. doi: 10.1128/jb.162.3.1100-1105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler I., Levinson H. S., Wang Y., Halvorson H. O. Cadmium- and mercury-resistant Bacillus strains from a salt marsh and from Boston Harbor. Appl Environ Microbiol. 1986 Dec;52(6):1293–1298. doi: 10.1128/aem.52.6.1293-1298.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner P. S., Falkinham J. O., 3rd Plasmid-encoded mercuric reductase in Mycobacterium scrofulaceum. J Bacteriol. 1984 Feb;157(2):669–672. doi: 10.1128/jb.157.2.669-672.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetaz T. J., Luke R. K. Plasmid-controlled resistance to copper in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1263–1268. doi: 10.1128/jb.154.3.1263-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Gos Z., Zajac J. Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J Bacteriol. 1981 Aug;147(2):305–312. doi: 10.1128/jb.147.2.305-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDER F. G., O'HARA C. Effects of iron deficiency and of zinc deficiency on the composition of Mycobacterium smegmatis. Biochem J. 1962 Jan;82:98–108. doi: 10.1042/bj0820098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Silver S., Kinscherf T. G. Cation transport alteration associated with plasmid-determined resistance to cadmium in Staphylococcus aureus. Antimicrob Agents Chemother. 1978 Dec;14(6):856–865. doi: 10.1128/aac.14.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]


