Abstract
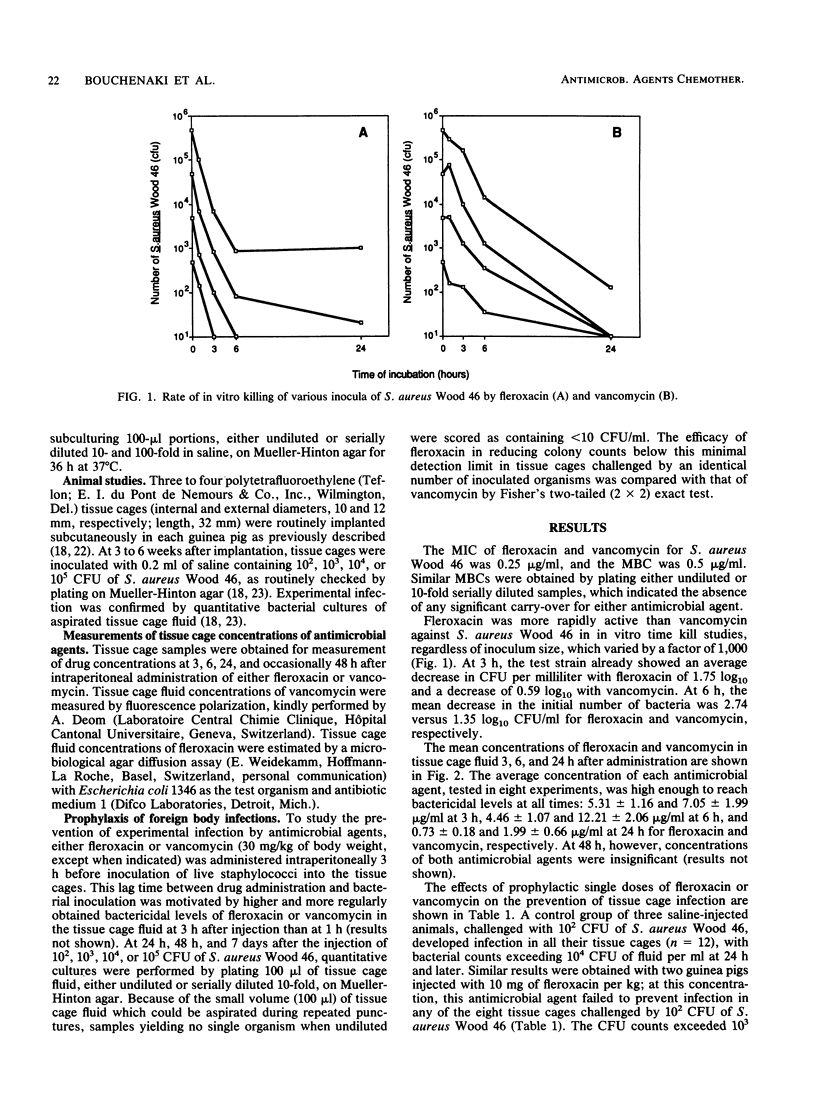
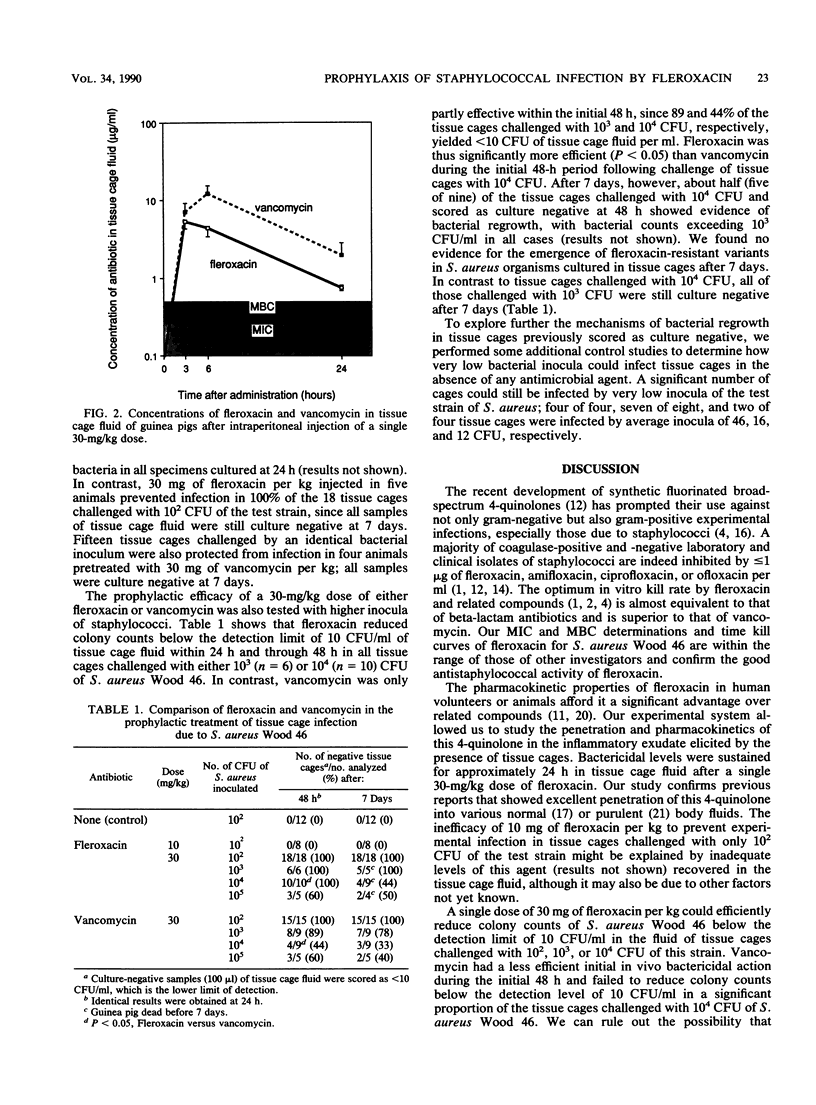
Single-dose administration of fleroxacin was evaluated as a means of preventing foreign body infection due to staphylococci. Tissue cages were implanted into guinea pigs and subsequently infected (100% rate) with 10(2) or more CFU of Staphylococcus aureus Wood 46. When a single dose of 30 mg of fleroxacin or vancomycin per kg of body weight was administered intraperitoneally, bactericidal levels of the antimicrobial agent were found in the tissue cage fluid after 3 h (when guinea pigs were inoculated with S. aureus) and during the next 24 h. Either fleroxacin or vancomycin successfully prevented experimental infection in all tissue cages challenged by 10(2) CFU of S. aureus Wood 46. When tissue cages were challenged with 10(4) CFU of S. aureus Wood 46, however, fleroxacin was more effective than vancomycin (P less than 0.05) in reducing colony counts below the detection limit of 10 CFU/ml in the inflammatory fluid of all tissue cages during the initial 48 h. In contrast to their initially different actions, the effects of the antibiotics were similar after 7 days, mostly because bacterial regrowth occurred more frequently in the fleroxacin-treated than in the vancomycin-treated tissue cages. These data show that experimental infections of subcutaneous tissue cages are a useful model for studying the prophylaxis of foreign body infections with antimicrobial agents.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Inoue M., Mitsuhashi S. In-vitro and in-vivo antibacterial activity of fleroxacin, a new fluorinated quinolone. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):99–114. doi: 10.1093/jac/22.supplement_d.99. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Eberlein E., Hörl G. Bactericidal kinetics of various dosages of fleroxacin simulated in bacterial cultures. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):81–89. doi: 10.1093/jac/22.supplement_d.81. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guerrero M., Rouse M., Henry N., Wilson W. Ciprofloxacin therapy of experimental endocarditis caused by methicillin-susceptible or methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1988 May;32(5):747–751. doi: 10.1128/aac.32.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina A. G., Costerton J. W. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis. J Bone Joint Surg Am. 1985 Feb;67(2):264–273. [PubMed] [Google Scholar]
- Nakashima M., Kanamaru M., Uematsu T., Takiguchi A., Mizuno A., Itaya T., Kawahara F., Ooie T., Saito S., Uchida H. Clinical pharmacokinetics and tolerance of fleroxacin in healthy male volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):133–144. doi: 10.1093/jac/22.supplement_d.133. [DOI] [PubMed] [Google Scholar]
- Paganoni R., Herzog C., Braunsteiner A., Hohl P. Fleroxacin: in-vitro activity worldwide against 20,807 clinical isolates and comparison to ciprofloxacin and norfloxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):3–17. doi: 10.1093/jac/22.supplement_d.3. [DOI] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D., Somerville M. M. In-vitro susceptibility of staphylococci to fleroxacin in comparison with six other quinolones. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):35–41. doi: 10.1093/jac/22.supplement_d.35. [DOI] [PubMed] [Google Scholar]
- Sande M. A., Brooks-Fournier R. A., Gerberding J. L. Efficacy of ciprofloxacin in animal models of infection: endocarditis, meningitis, and pneumonia. Am J Med. 1987 Apr 27;82(4A):63–66. [PubMed] [Google Scholar]
- Sorgel F., Metz R., Naber K., Seelmann R., Muth P. Pharmacokinetics and body fluid penetration of fleroxacin in healthy volunteers. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):155–167. doi: 10.1093/jac/22.supplement_d.155. [DOI] [PubMed] [Google Scholar]
- Tshefu K., Zimmerli W., Waldvogel F. A. Short-term administration of rifampin in the prevention or eradication of infection due to foreign bodies. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S474–S480. doi: 10.1093/clinids/5.supplement_3.s474. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Suzuki R., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984 Oct;150(4):546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- Weidekamm E., Portmann R., Partos C., Dell D. Single and multiple dose pharmacokinetics of fleroxacin. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):145–154. doi: 10.1093/jac/22.supplement_d.145. [DOI] [PubMed] [Google Scholar]
- Youngs D. J., Tudor R. G., Yoshioka K., Keighley M. R. The penetration of fleroxacin into intra-abdominal abscesses. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):115–118. doi: 10.1093/jac/22.supplement_d.115. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Waldvogel F. A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest. 1984 Apr;73(4):1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Zak O., Vosbeck K. Experimental hematogenous infection of subcutaneously implanted foreign bodies. Scand J Infect Dis. 1985;17(3):303–310. doi: 10.3109/inf.1985.17.issue-3.10. [DOI] [PubMed] [Google Scholar]



