Abstract
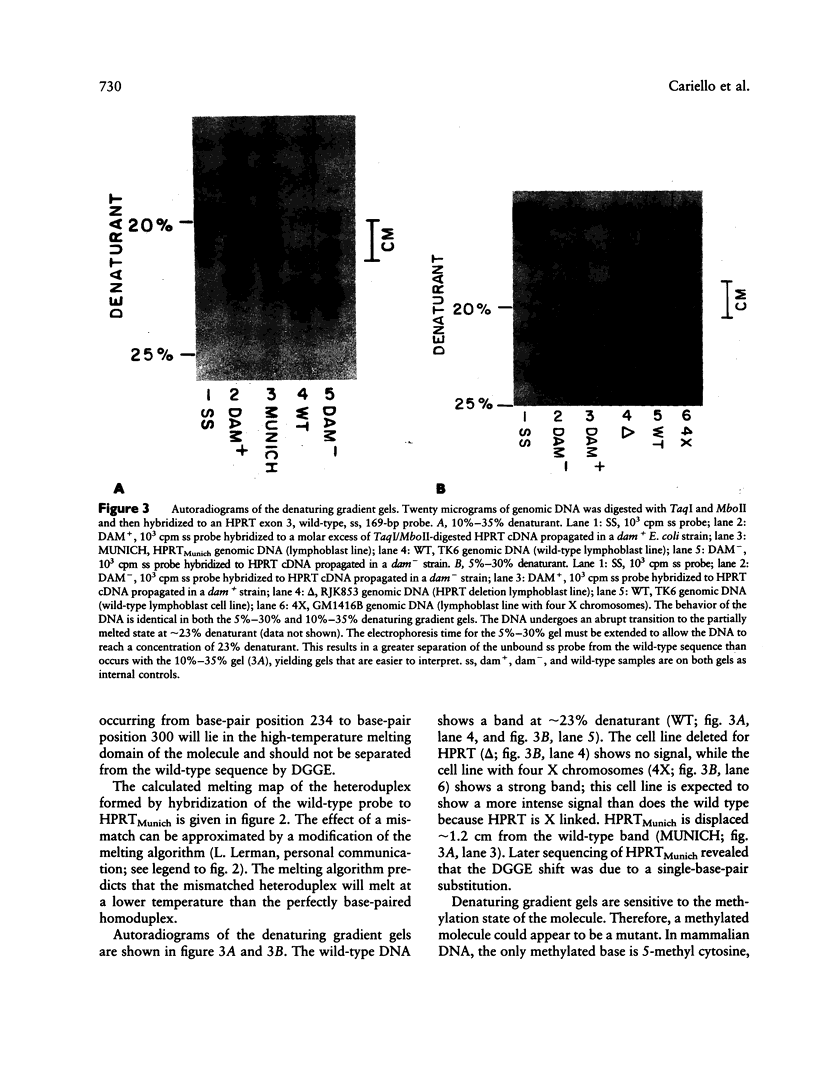
The combination of denaturing gradient gel electrophoresis (DGGE) and in vitro DNA amplification has allowed us to (1) localize a DNA mutation to a given 100-bp region of the human genome and (2) rapidly sequence the DNA without cloning. DGGE showed that a mutation had occurred, but the technique revealed little about the nature or position of that mutation. The region of the genome containing the mutation was amplified by the polymerase chain-reaction technique, providing DNA of sufficient quality and quantity for direct sequencing. Amplification was performed with a 32P end-labeled primer that allowed direct Maxam-Gilbert sequencing of the amplified product without cloning. HPRTMunich was found to contain a single-base-pair substitution, a C-to-A transversion at base-pair position 397. We report the generation of a 169-bp, wild-type DNA probe that encompasses most of exon 3 of the human hypoxanthine guanine phosphoribosyltransferase (HPRT) gene and contains a low-temperature melting domain of approximately 100 bp. HPRTMunich, an HPRT mutant isolated from a patient with gout, has a single amino acid substitution; the corresponding DNA sequence alteration must lie within the low-temperature melting domain of exon 3. We report the separation of HPRTMunich from the wild-type sequence using DGGE. In addition to base-pair substitutions, DGGE is also sensitive to the methylation state of the molecule. The cDNA for HPRT was cloned into a vector and propagated in Escherichia coli dam+ and dam- strains; thus, methylated and unmethylated HPRT cDNA was obtained.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariello N. F., Thilly W. G. Use of gradient denaturing gels to determine mutational spectrum in human cells. Basic Life Sci. 1986;38:439–452. doi: 10.1007/978-1-4615-9462-8_46. [DOI] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Keohavong P., Kat A. G., Cariello N. F., Thilly W. G. DNA amplification in vitro using T4 DNA polymerase. DNA. 1988 Jan-Feb;7(1):63–70. doi: 10.1089/dna.1988.7.63. [DOI] [PubMed] [Google Scholar]
- Kessler C., Neumaier P. S., Wolf W. Recognition sequences of restriction endonucleases and methylases--a review. Gene. 1985;33(1):1–102. doi: 10.1016/0378-1119(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Fischer S. G., Hurley I., Silverstein K., Lumelsky N. Sequence-determined DNA separations. Annu Rev Biophys Bioeng. 1984;13:399–423. doi: 10.1146/annurev.bb.13.060184.002151. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K., Grinfeld E. Searching for gene defects by denaturing gradient gel electrophoresis. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):285–297. doi: 10.1101/sqb.1986.051.01.034. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMahon G., Davis E., Wogan G. N. Characterization of c-Ki-ras oncogene alleles by direct sequencing of enzymatically amplified DNA from carcinogen-induced tumors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4974–4978. doi: 10.1073/pnas.84.14.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Maniatis T., Lerman L. S. Modification of the melting properties of duplex DNA by attachment of a GC-rich DNA sequence as determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3111–3129. doi: 10.1093/nar/13.9.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T. Recent advances in the development of methods for detecting single-base substitutions associated with human genetic diseases. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):275–284. doi: 10.1101/sqb.1986.051.01.033. [DOI] [PubMed] [Google Scholar]
- Noll W. W., Collins M. Detection of human DNA polymorphisms with a simplified denaturing gradient gel electrophoresis technique. Proc Natl Acad Sci U S A. 1987 May;84(10):3339–3343. doi: 10.1073/pnas.84.10.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Nussbaum R. L., gramson P. E., Ledbetter D. H., Caskey C. T., Chinault A. C. Organization of the HPRT gene and related sequences in the human genome. Somat Cell Mol Genet. 1984 Sep;10(5):483–493. doi: 10.1007/BF01534853. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Penman B. W., Thilly W. G. Isolation of a human lymphoblastoid line heterozygous at the thymidine kinase locus: possibility for a rapid human cell mutation assay. Biochem Biophys Res Commun. 1978 Sep 29;84(2):411–416. doi: 10.1016/0006-291x(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. HPRT: gene structure, expression, and mutation. Annu Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- Thilly W. G. Potential use of gradient denaturing gel electrophoresis in obtaining mutational spectra from human cells. Carcinog Compr Surv. 1985;10:511–528. [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. Recognition and estimation of 5-methylcytosine in nucleic acids. Biochem J. 1951 May;48(5):581–584. doi: 10.1042/bj0480581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Structural alteration in a dysfunctional enzyme variant (HPRTMunich) isolated from a patient with gout. J Biol Chem. 1984 Jan 10;259(1):27–30. [PubMed] [Google Scholar]
- Wilson J. M., Young A. B., Kelley W. N. Hypoxanthine-guanine phosphoribosyltransferase deficiency. The molecular basis of the clinical syndromes. N Engl J Med. 1983 Oct 13;309(15):900–910. doi: 10.1056/NEJM198310133091507. [DOI] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]