Abstract
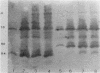
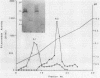
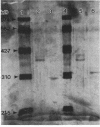
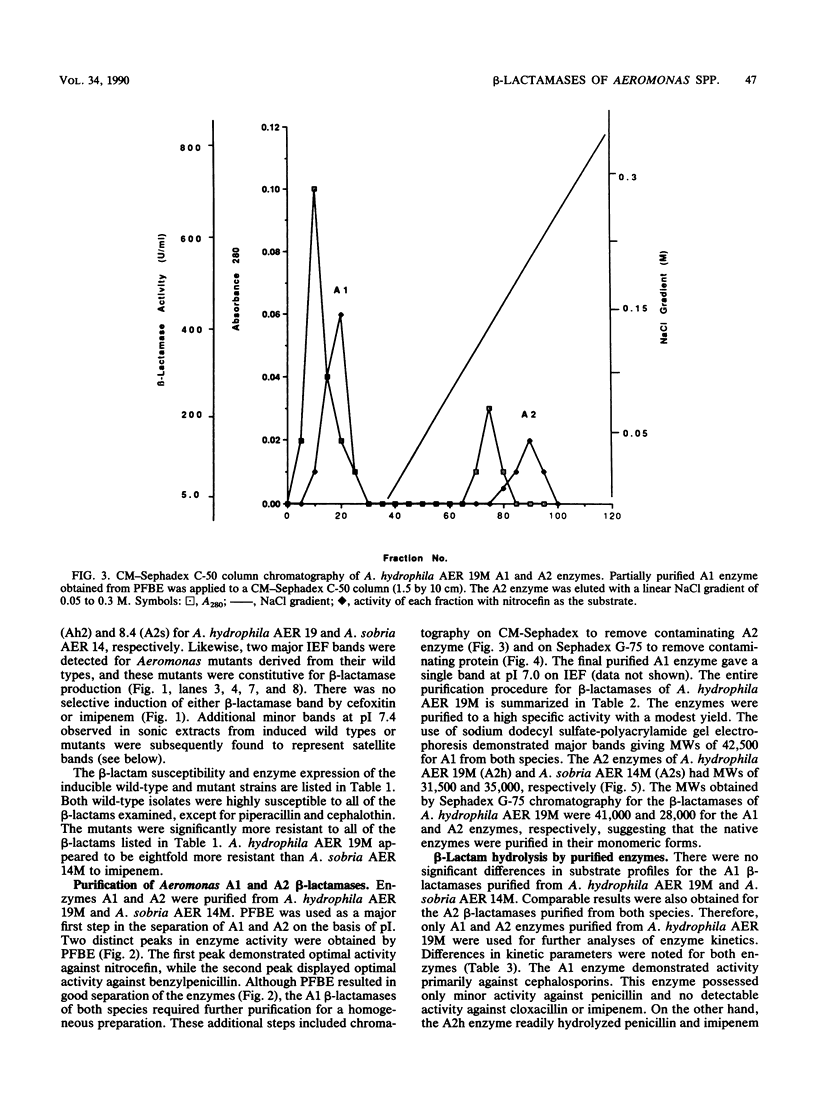
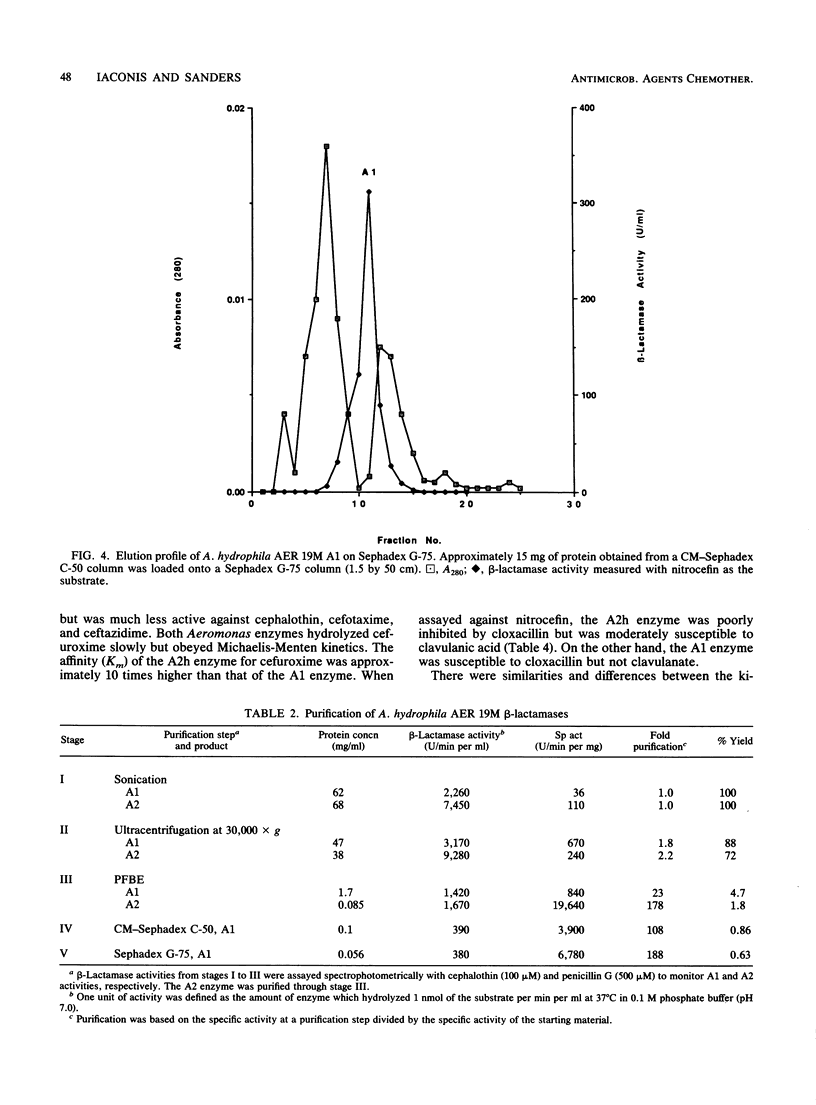
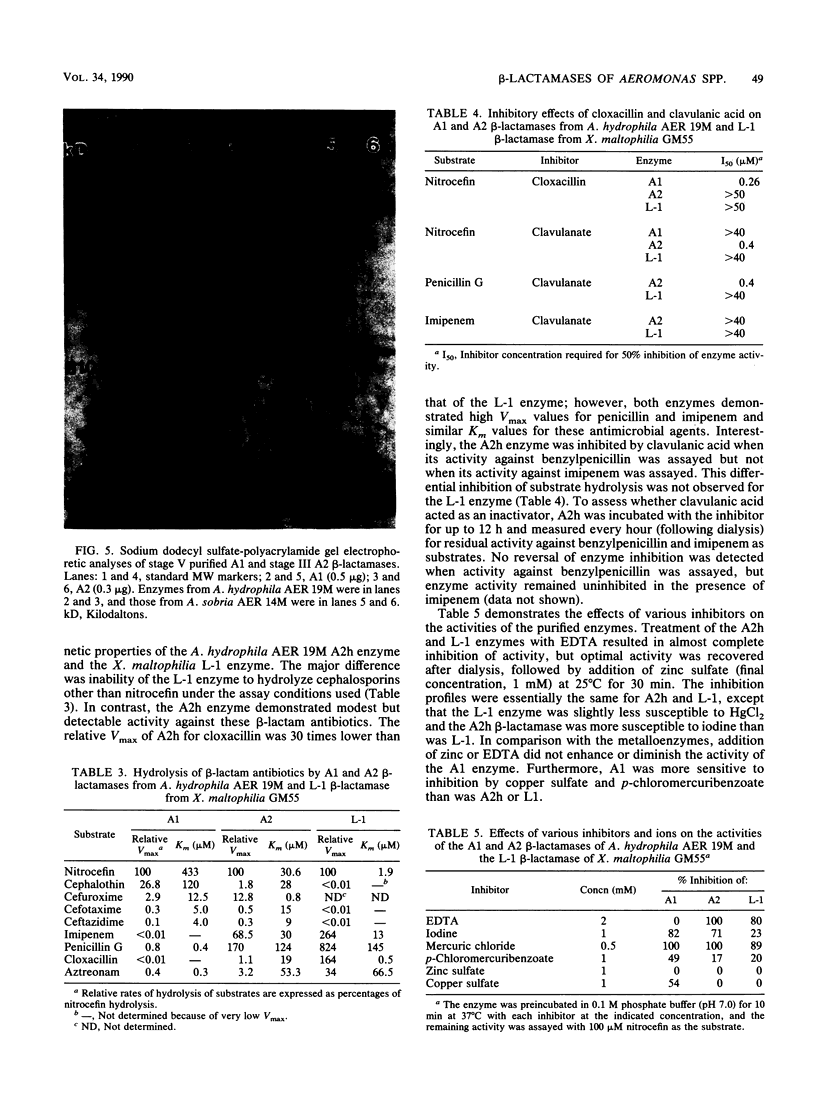
beta-Lactamases from Aeromonas hydrophila and A. sobria were purified and characterized. Both species produced beta-lactamases that were inducible by either cefoxitin or imipenem. These species were resistant to ampicillin and cephalothin but not imipenem. Isoelectric focusing of sonic extracts revealed one band at pI 8.0 and a second band at pI 7.0 for A. hydrophila. Likewise, A. sobria produced two bands, one at pI 8.4 and the other at pI 7.0. Two enzymes from each species were separated by flatbed electrofocusing gel and purified to homogeneity. The molecular weight of the pI 7.0 enzyme (A1) from both species was estimated to be 42,500, whereas the pI 8.0 (A2h) and 8.4 (A2s) enzymes of A. hydrophila and A. sobria had molecular weights of 31,500 and 35,000, respectively, on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The relative Vmax values for cephalothin, penicillin, and imipenem for these enzymes indicated that A1 was primarily a cephalosporinase while A2h and A2s were penicillinases highly active against carbapenems. A1 was susceptible to inhibition by cloxacillin, while the A2 enzymes were inhibited by clavulanic acid and EDTA and required zinc for activity. Thus, there appear to be two distinct inducible beta-lactamases in A. hydrophila and A. sobria that play an important role in the beta-lactam resistance of these species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakken J. S., Sanders C. C., Clark R. B., Hori M. Beta-lactam resistance in Aeromonas spp. caused by inducible beta-lactamases active against penicillins, cephalosporins, and carbapenems. Antimicrob Agents Chemother. 1988 Sep;32(9):1314–1319. doi: 10.1128/aac.32.9.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy M., Péduzzi J., Ben Yaghlane H., Labia R. Single amino acid substitution between SHV-1 beta-lactamase and cefotaxime-hydrolyzing SHV-2 enzyme. FEBS Lett. 1988 Apr 11;231(1):217–220. doi: 10.1016/0014-5793(88)80734-8. [DOI] [PubMed] [Google Scholar]
- Bateman J. L., Tu R. P., Strampfer M. J., Cunha B. A. Aeromonas hydrophila cellulitis and wound infections caused by waterborne organisms. Heart Lung. 1988 Jan;17(1):99–102. [PubMed] [Google Scholar]
- Bicknell R., Emanuel E. L., Gagnon J., Waley S. G. The production and molecular properties of the zinc beta-lactamase of Pseudomonas maltophilia IID 1275. Biochem J. 1985 Aug 1;229(3):791–797. doi: 10.1042/bj2290791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Waley S. G. Cryoenzymology of Bacillus cereus beta-lactamase II. Biochemistry. 1985 Nov 19;24(24):6876–6887. doi: 10.1021/bi00345a021. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 1, 2a, 2b, and 2b'. Antimicrob Agents Chemother. 1989 Mar;33(3):264–270. doi: 10.1128/aac.33.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Sykes R. B. beta-Lactamase inhibitors in perspective. J Antimicrob Chemother. 1983 Feb;11(2):97–107. doi: 10.1093/jac/11.2.97. [DOI] [PubMed] [Google Scholar]
- Challapalli M., Tess B. R., Cunningham D. G., Chopra A. K., Houston C. W. Aeromonas-associated diarrhea in children. Pediatr Infect Dis J. 1988 Oct;7(10):693–698. doi: 10.1097/00006454-198810000-00005. [DOI] [PubMed] [Google Scholar]
- Chang B. J., Bolton S. M. Plasmids and resistance to antimicrobial agents in Aeromonas sobria and Aeromonas hydrophila clinical isolates. Antimicrob Agents Chemother. 1987 Aug;31(8):1281–1282. doi: 10.1128/aac.31.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnas R. L., Fisher J., Knowles J. R. Chemical studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2185–2189. doi: 10.1021/bi00604a025. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Abraham E. P. Beta-lactamases from Yersinia enterocolitica. J Gen Microbiol. 1975 Apr;87(2):273–284. doi: 10.1099/00221287-87-2-273. [DOI] [PubMed] [Google Scholar]
- Díaz A., Velasco A. C., Hawkins F., Cabañas F. Aeromonas hydrophila-associated diarrhea in a neonate. Pediatr Infect Dis. 1986 Nov-Dec;5(6):704–704. doi: 10.1097/00006454-198611000-00022. [DOI] [PubMed] [Google Scholar]
- Fujii T., Sato K., Miyata K., Inoue M., Mitsuhashi S. Biochemical properties of beta-lactamase produced by Legionella gormanii. Antimicrob Agents Chemother. 1986 May;29(5):925–926. doi: 10.1128/aac.29.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Medeiros A. A., Cohenford M., Jacoby G. A. Genetic and biochemical properties of AER-1, a novel carbenicillin-hydrolyzing beta-lactamase from Aeromonas hydrophila. Antimicrob Agents Chemother. 1985 Apr;27(4):479–484. doi: 10.1128/aac.27.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman-Brenner F. W., MacDonald K. L., Steigerwalt A. G., Fanning G. R., Brenner D. J., Farmer J. J., 3rd Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J Clin Microbiol. 1987 May;25(5):900–906. doi: 10.1128/jcm.25.5.900-906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs R. D., Paviour S. D., Bunker D. E., Lang S. D. Wound infection with aerogenic Aeromonas strains: a review of twenty-seven cases. Eur J Clin Microbiol Infect Dis. 1988 Jun;7(3):355–360. doi: 10.1007/BF01962336. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Duffey P. S. Mesophilic aeromonads in human disease: current taxonomy, laboratory identification, and infectious disease spectrum. Rev Infect Dis. 1988 Sep-Oct;10(5):980–997. doi: 10.1093/clinids/10.5.980. [DOI] [PubMed] [Google Scholar]
- Kuijper E. J., Steigerwalt A. G., Schoenmakers B. S., Peeters M. F., Zanen H. C., Brenner D. J. Phenotypic characterization and DNA relatedness in human fecal isolates of Aeromonas spp. J Clin Microbiol. 1989 Jan;27(1):132–138. doi: 10.1128/jcm.27.1.132-138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp W. L., Borden D. G. Further Studies on Proteus hydrophilus, the Etiological Agent in "Red Leg" Disease of Frogs. J Bacteriol. 1942 Dec;44(6):673–685. doi: 10.1128/jb.44.6.673-685.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M. Plasmid-mediated beta-lactamases of Gram-negative bacteria: properties and distribution. J Antimicrob Chemother. 1979 Jul;5(4):349–358. doi: 10.1093/jac/5.4.349. [DOI] [PubMed] [Google Scholar]
- Motyl M. R., McKinley G., Janda J. M. In vitro susceptibilities of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae to 22 antimicrobial agents. Antimicrob Agents Chemother. 1985 Jul;28(1):151–153. doi: 10.1128/aac.28.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto T. P., Ellis A. E. Characterization of extracellular metallo- and serine-proteases of Aeromonas hydrophila strain B51. J Gen Microbiol. 1986 Jul;132(7):1975–1979. doi: 10.1099/00221287-132-7-1975. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Abraham E. P. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J. 1966 Jan;98(1):11C–13C. doi: 10.1042/bj0980011c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Joaquin V. H., Scribner R. K., Pickett D. A., Welch D. F. Antimicrobial susceptibility of Aeromonas species isolated from patients with diarrhea. Antimicrob Agents Chemother. 1986 Nov;30(5):794–795. doi: 10.1128/aac.30.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Fujii T., Okamoto R., Inoue M., Mitsuhashi S. Biochemical properties of beta-lactamase produced by Flavobacterium odoratum. Antimicrob Agents Chemother. 1985 Apr;27(4):612–614. doi: 10.1128/aac.27.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Morioka K., Ogawa M., Yamagishi S. Inducible oxacillin-hydrolyzing penicillinase in Aeromonas hydrophila isolated from fish. Antimicrob Agents Chemother. 1976 Aug;10(2):191–195. doi: 10.1128/aac.10.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K., King A., Phillips I. Beta-lactamases with high activity against imipenem and Sch 34343 from Aeromonas hydrophila. J Antimicrob Chemother. 1986 Jan;17(1):45–50. doi: 10.1093/jac/17.1.45. [DOI] [PubMed] [Google Scholar]
- Steffen R., Mathewson J. J., Ericsson C. D., DuPont H. L., Helminger A., Balm T. K., Wolff K., Witassek F. Travelers' diarrhea in West Africa and Mexico: fecal transport systems and liquid bismuth subsalicylate for self-therapy. J Infect Dis. 1988 May;157(5):1008–1013. doi: 10.1093/infdis/157.5.1008. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Artymiuk P. J., Cordero-Borboa A. E., Little C., Phillips D. C., Waley S. G. An X-ray-crystallographic study of beta-lactamase II from Bacillus cereus at 0.35 nm resolution. Biochem J. 1987 Nov 15;248(1):181–188. doi: 10.1042/bj2480181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]