Abstract
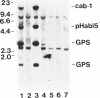
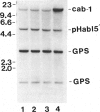
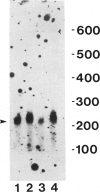
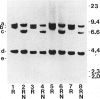
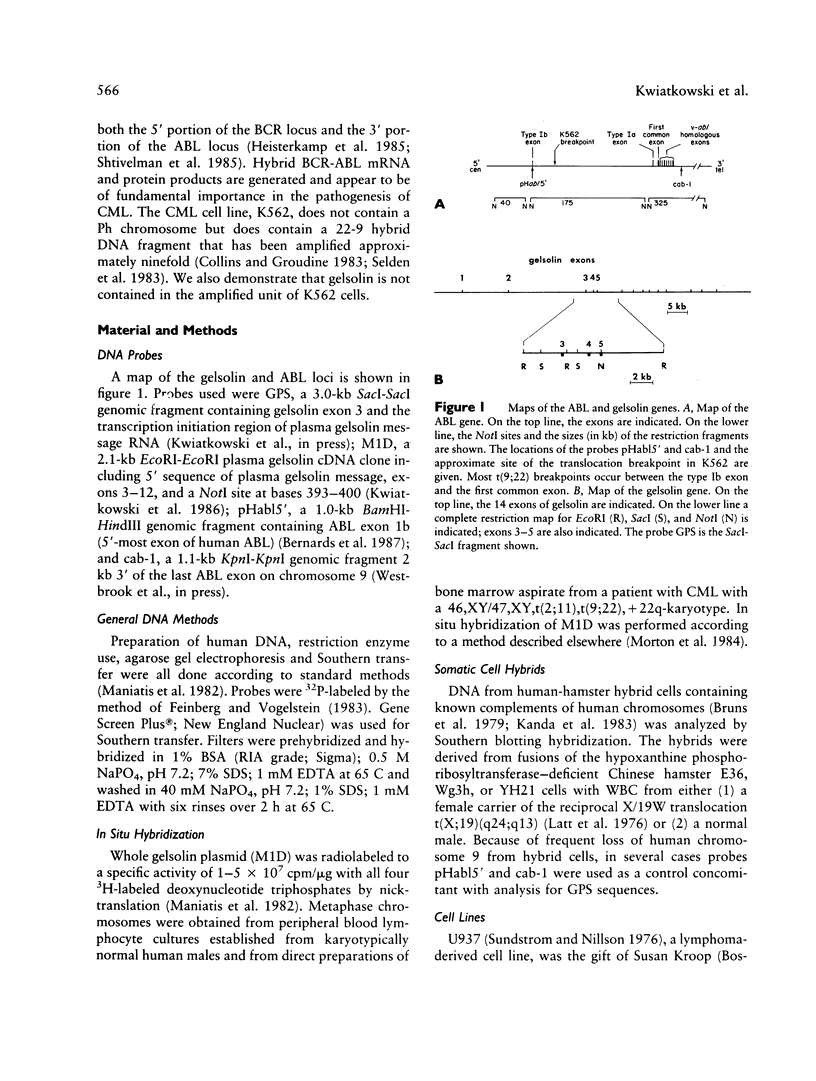
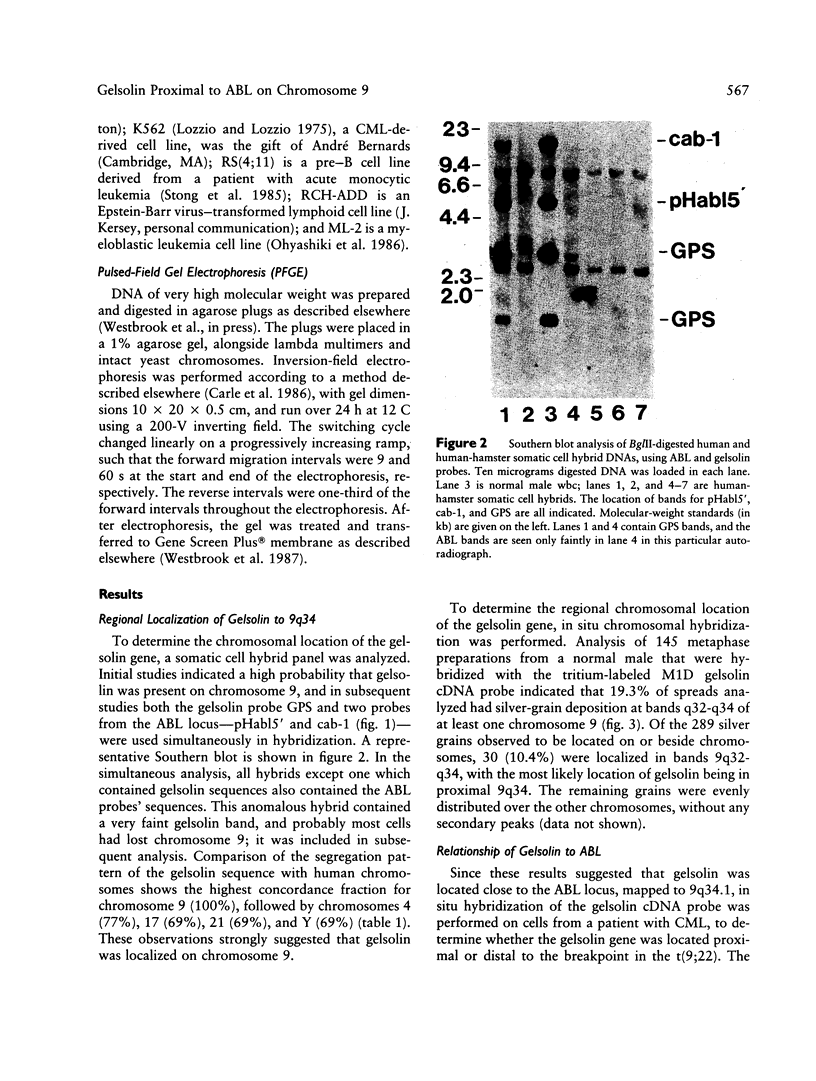
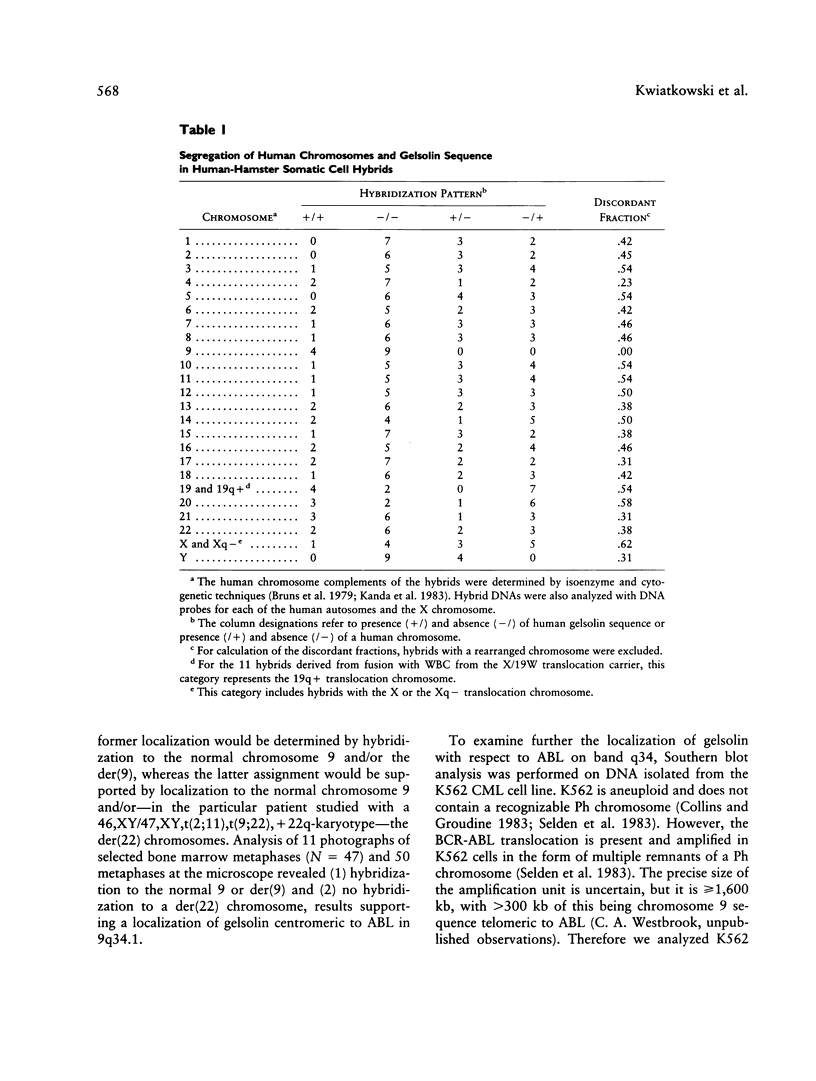
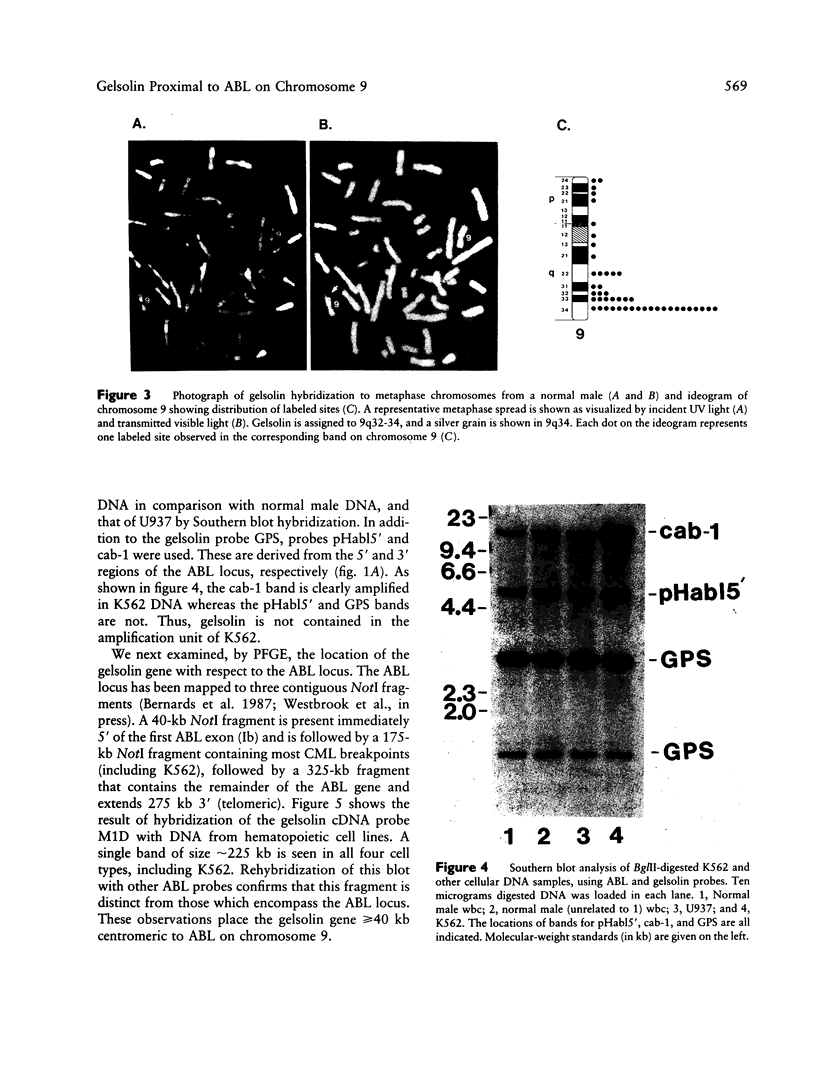
Gelsolin is a plasma and cytoskeletal protein that severs actin filaments and is regulated by both Ca+2 and polyphosphoinositides. The two forms of gelsolin are encoded by a single gene and derived through alternative message splicing. By Southern blot analysis of somatic cell hybrids and in situ chromosomal localization, we demonstrate that the gelsolin gene is present on human chromosome 9 in bands q32-q34. In situ hybridization of gelsolin to cells containing a Philadelphia chromosome [(9;22)(q34;q11)], as well as Southern blot analysis of K562 cell DNA, indicates that gelsolin is centromeric to the ABL locus in 9q34. Southern blot analysis of NotI-digested, pulsed-field gel electrophoresis-separated DNA indicates the gelsolin gene is greater than or equal to 40 kb centromeric to ABL. These studies and standard Southern blot analysis of digested DNA also indicate that the NotI restriction site contained in the gelsolin gene is uncleavable in DNA from white blood cells and hematopoietic cell lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Rubin C. M., Westbrook C. A., Paskind M., Baltimore D. The first intron in the human c-abl gene is at least 200 kilobases long and is a target for translocations in chronic myelogenous leukemia. Mol Cell Biol. 1987 Sep;7(9):3231–3236. doi: 10.1128/mcb.7.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Mintz B. J., Leary A. C., Regina V. M., Gerald P. S. Human lysosomal genes: arylsulfatase A and beta-galactosidase. Biochem Genet. 1979 Dec;17(11-12):1031–1059. doi: 10.1007/BF00504344. [DOI] [PubMed] [Google Scholar]
- Bryan J., Kurth M. C. Actin-gelsolin interactions. Evidence for two actin-binding sites. J Biol Chem. 1984 Jun 25;259(12):7480–7487. [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Borgia R., Rungger-Brändle E., Weil R., Gabbiani G. An actin-destabilizing factor is present in human plasma. Experientia. 1979 Aug 15;35(8):1039–1041. doi: 10.1007/BF01949928. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Groudine M. T. Rearrangement and amplification of c-abl sequences in the human chronic myelogenous leukemia cell line K-562. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4813–4817. doi: 10.1073/pnas.80.15.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. J., Robson E. B., Buckton K. E., Slaughter C. A., Gray J. E., Blank C. E., James F. E., Ridler M. A., Insley J., Hultén M. Segregation of ABO, AK1 and ACONs in families with abnormalities of chromosome 9. Ann Hum Genet. 1978 Jan;41(3):365–377. doi: 10.1111/j.1469-1809.1978.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R. The human v-abl cellular homologue. J Mol Appl Genet. 1983;2(1):57–68. [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Chaponnier C., Lind S. E., Zaner K. S., Stossel T. P., Yin H. L. Interactions of gelsolin and gelsolin-actin complexes with actin. Effects of calcium on actin nucleation, filament severing, and end blocking. Biochemistry. 1985 Jul 2;24(14):3714–3723. doi: 10.1021/bi00335a046. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Iida K., Yin H. L., Stossel T. P. Polyphosphoinositide micelles and polyphosphoinositide-containing vesicles dissociate endogenous gelsolin-actin complexes and promote actin assembly from the fast-growing end of actin filaments blocked by gelsolin. J Biol Chem. 1987 Sep 5;262(25):12228–12236. [PubMed] [Google Scholar]
- Janmey P. A., Stossel T. P. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987 Jan 22;325(6102):362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- Kanda N., Schreck R., Alt F., Bruns G., Baltimore D., Latt S. Isolation of amplified DNA sequences from IMR-32 human neuroblastoma cells: facilitation by fluorescence-activated flow sorting of metaphase chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4069–4073. doi: 10.1073/pnas.80.13.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Stossel T. P., Orkin S. H., Mole J. E., Colten H. R., Yin H. L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986 Oct 2;323(6087):455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Willard H. F., Gerald P. S. BrdU-33258 Hoechst analysis of DNA replication in human lymphocytes with supernumerary or structurally abnormal X chromosomes. Chromosoma. 1976 Aug 17;57(2):135–153. doi: 10.1007/BF00292912. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Morton C. C., Kirsch I. R., Taub R., Orkin S. H., Brown J. A. Localization of the beta-globin gene by chromosomal in situ hybridization. Am J Hum Genet. 1984 May;36(3):576–585. [PMC free article] [PubMed] [Google Scholar]
- Norberg R., Thorstensson R., Utter G., Fagraeus A. F-Actin-depolymerizing activity of human serum. Eur J Biochem. 1979 Oct 15;100(2):575–583. doi: 10.1111/j.1432-1033.1979.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Ohyashiki K., Ohyashiki J. H., Sandberg A. A. Cytogenetic characterization of putative human myeloblastic leukemia cell lines (ML-1, -2, and -3): origin of the cells. Cancer Res. 1986 Jul;46(7):3642–3647. [PubMed] [Google Scholar]
- Rowley J. D. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973 Jun 1;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- Rowley J. D., Testa J. R. Chromosome abnormalities in malignant hematologic diseases. Adv Cancer Res. 1982;36:103–148. doi: 10.1016/s0065-230x(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Selden J. R., Emanuel B. S., Wang E., Cannizzaro L., Palumbo A., Erikson J., Nowell P. C., Rovera G., Croce C. M. Amplified C lambda and c-abl genes are on the same marker chromosome in K562 leukemia cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7289–7292. doi: 10.1073/pnas.80.23.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Janmey P. A., Herbert T. J., Lind S. E. Quantitative measurement of plasma gelsolin and its incorporation into fibrin clots. J Lab Clin Med. 1987 Aug;110(2):189–195. [PubMed] [Google Scholar]
- Stong R. C., Korsmeyer S. J., Parkin J. L., Arthur D. C., Kersey J. H. Human acute leukemia cell line with the t(4;11) chromosomal rearrangement exhibits B lineage and monocytic characteristics. Blood. 1985 Jan;65(1):21–31. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Westbrook C. A., Rubin C. M., Le Beau M. M., Kaminer L. S., Smith S. D., Rowley J. D., Diaz M. O. Molecular analysis of TCRB and ABL in a t(7;9)-containing cell line (SUP-T3) from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1987 Jan;84(1):251–255. doi: 10.1073/pnas.84.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Albrecht J. H., Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Kwiatkowski D. J., Mole J. E., Cole F. S. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J Biol Chem. 1984 Apr 25;259(8):5271–5276. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]