Abstract
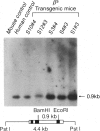
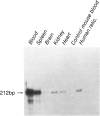
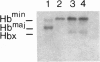
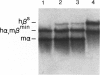
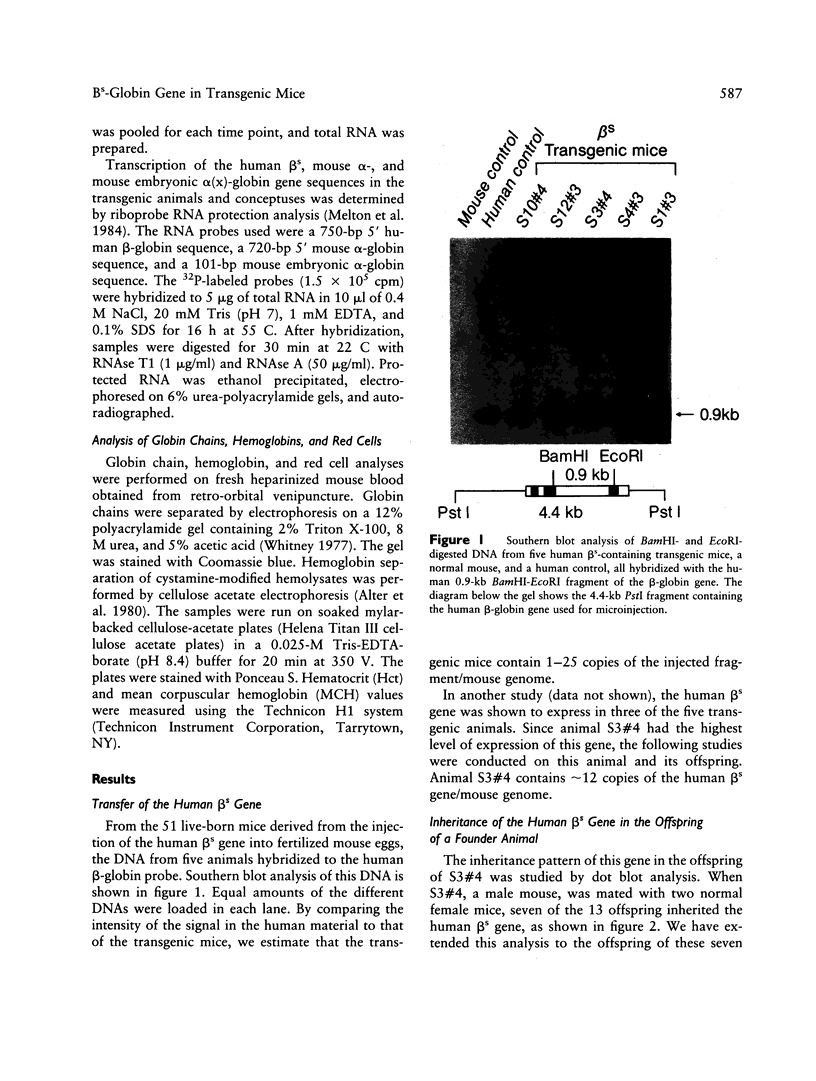
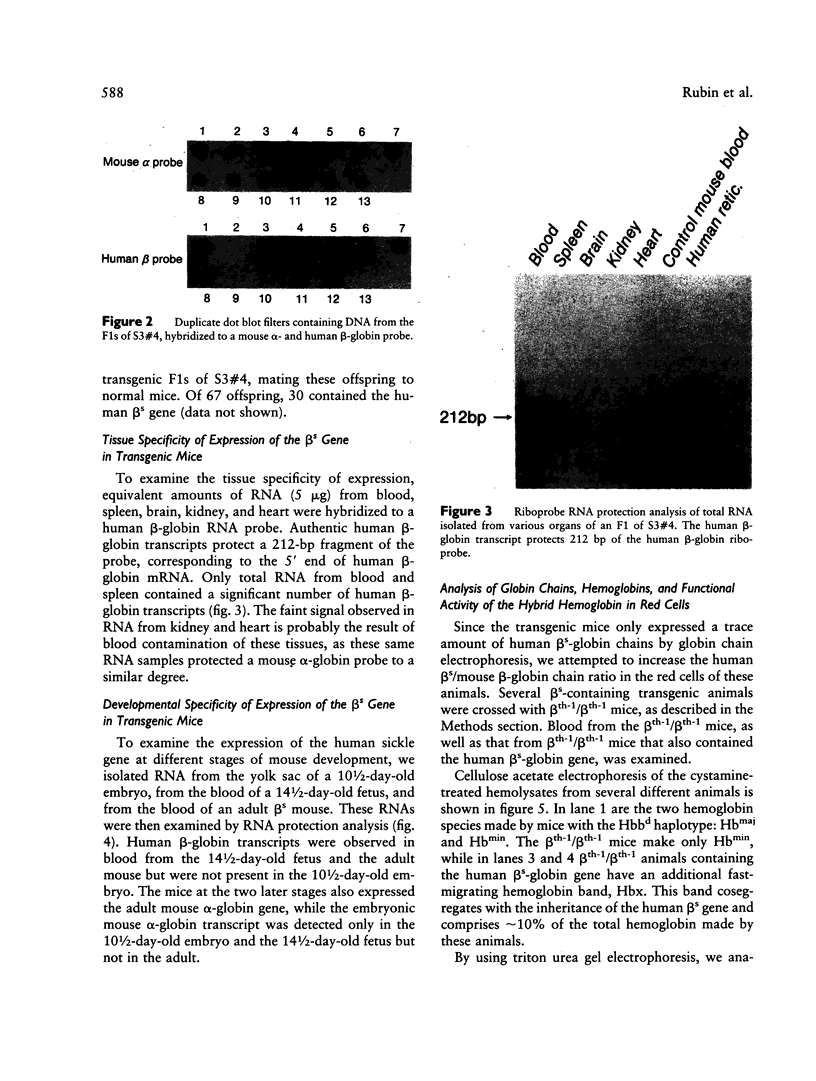
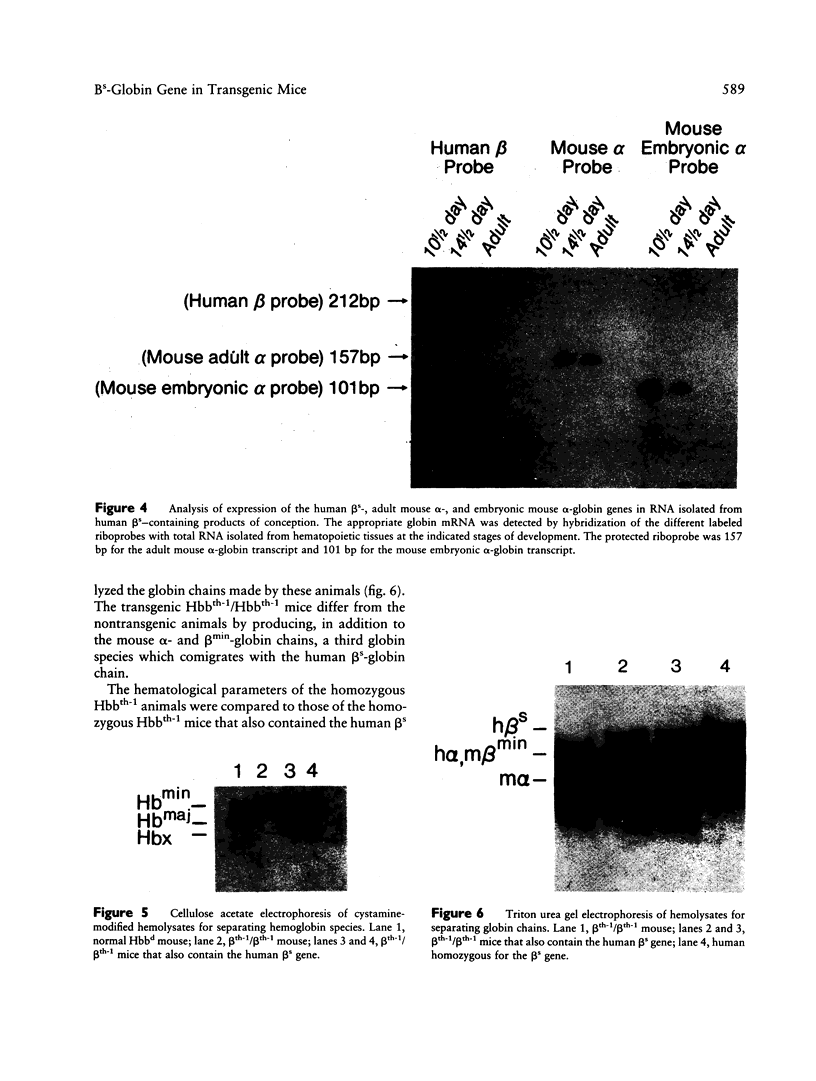
Owing to the episodic and unpredictable nature of the sickling crisis, many aspects of the disease sickle cell anemia have resisted in vivo analysis. The lack of an animal model has hindered the pathophysiological investigation of this disease, as well as deterred the development of pharmacological therapies. The transgenic mouse system offers a new means for creating animals that make a specified mutant gene product, and we have used this system to create a series of mice that contain the human beta s-globin gene. These animals express this gene in the appropriate tissues and at the same point in development as the adult mouse globin genes are expressed. We have crossed the human beta s-containing transgenic mice with a beta-thalassemic mouse line and examined the hemoglobins produced by these mice. Their red cells contain 10% mouse alpha/human beta s hybrid hemoglobin, which partially corrects the thalassemic phenotype of the homozygous beta-thalassemic animals. Though the red cells do not sickle, other properties of the human beta s gene in these mice indicate the potential for the eventual development of a transgenic animal model for sickle cell anemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Castro O., Finch S. C., Osbaldiston G. Sickle cell resistance to in vivo hypoxia. Nature. 1974 Oct 18;251(5476):620–621. doi: 10.1038/251620a0. [DOI] [PubMed] [Google Scholar]
- Chada K., Magram J., Raphael K., Radice G., Lacy E., Costantini F. Specific expression of a foreign beta-globin gene in erythroid cells of transgenic mice. 1985 Mar 28-Apr 3Nature. 314(6009):377–380. doi: 10.1038/314377a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Costantini F., Chada K., Magram J. Correction of murine beta-thalassemia by gene transfer into the germ line. Science. 1986 Sep 12;233(4769):1192–1194. doi: 10.1126/science.3461564. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Kollias G., Wrighton N., Hurst J., Grosveld F. Regulated expression of human A gamma-, beta-, and hybrid gamma beta-globin genes in transgenic mice: manipulation of the developmental expression patterns. Cell. 1986 Jul 4;46(1):89–94. doi: 10.1016/0092-8674(86)90862-7. [DOI] [PubMed] [Google Scholar]
- Magram J., Chada K., Costantini F. Developmental regulation of a cloned adult beta-globin gene in transgenic mice. Nature. 1985 May 23;315(6017):338–340. doi: 10.1038/315338a0. [DOI] [PubMed] [Google Scholar]
- Martinell J., Whitney J. B., 3rd, Popp R. A., Russell L. B., Anderson W. F. Three mouse models of human thalassemia. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5056–5060. doi: 10.1073/pnas.78.8.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monplaisir N., Merault G., Poyart C., Rhoda M. D., Craescu C., Vidaud M., Galacteros F., Blouquit Y., Rosa J. Hemoglobin S Antilles: a variant with lower solubility than hemoglobin S and producing sickle cell disease in heterozygotes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9363–9367. doi: 10.1073/pnas.83.24.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. B., 3rd Simplified typing of mouse hemoglobin (Hbb) phenotypes using cystamine. Biochem Genet. 1978 Aug;16(7-8):667–672. doi: 10.1007/BF00484723. [DOI] [PubMed] [Google Scholar]