Abstract
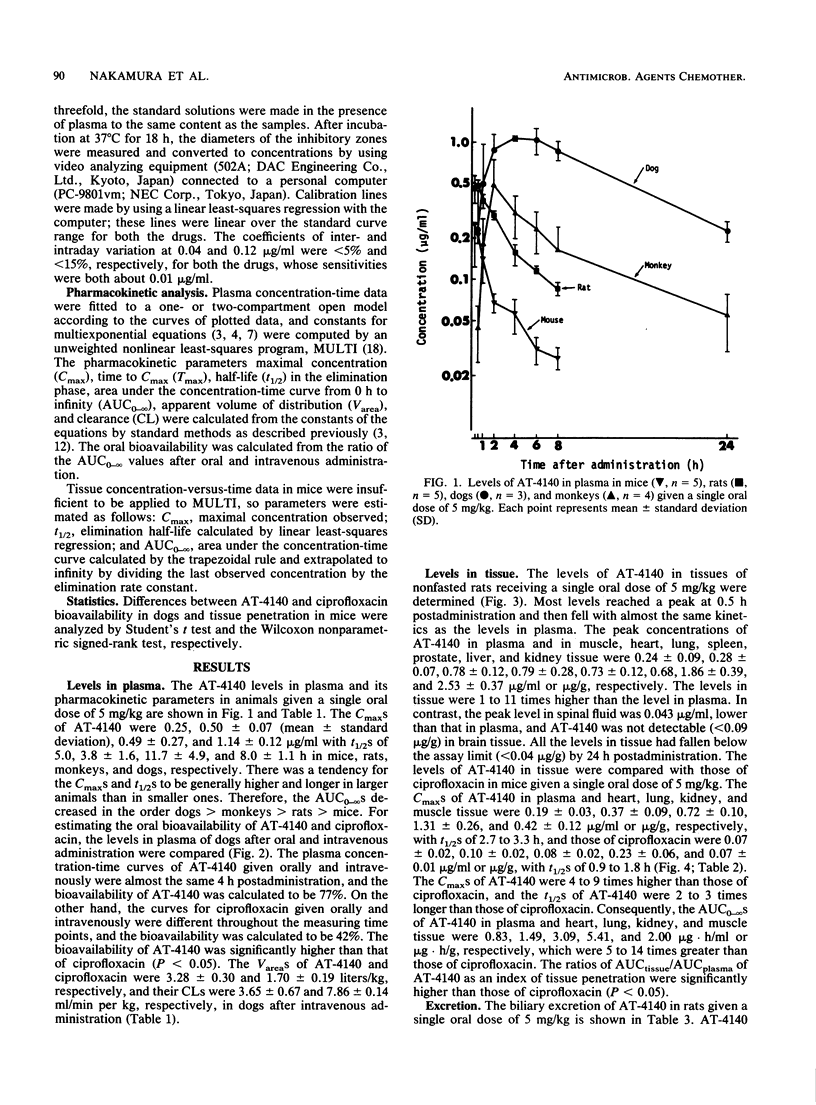
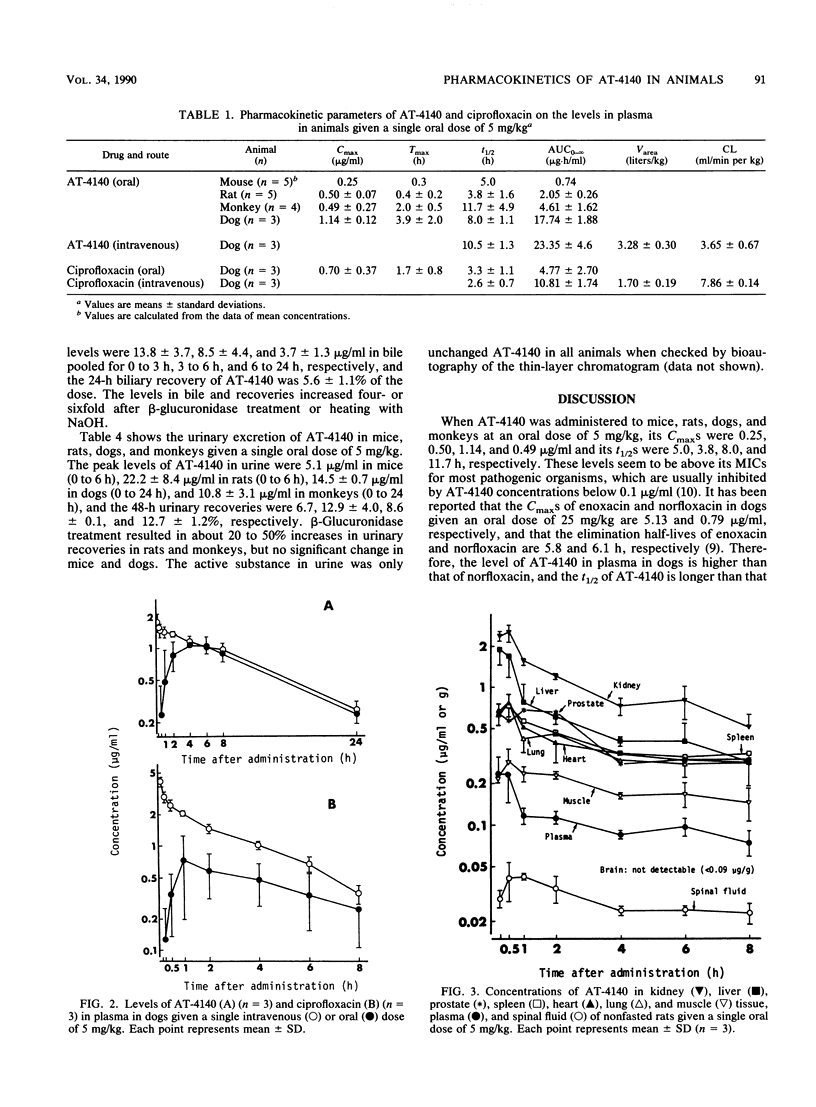
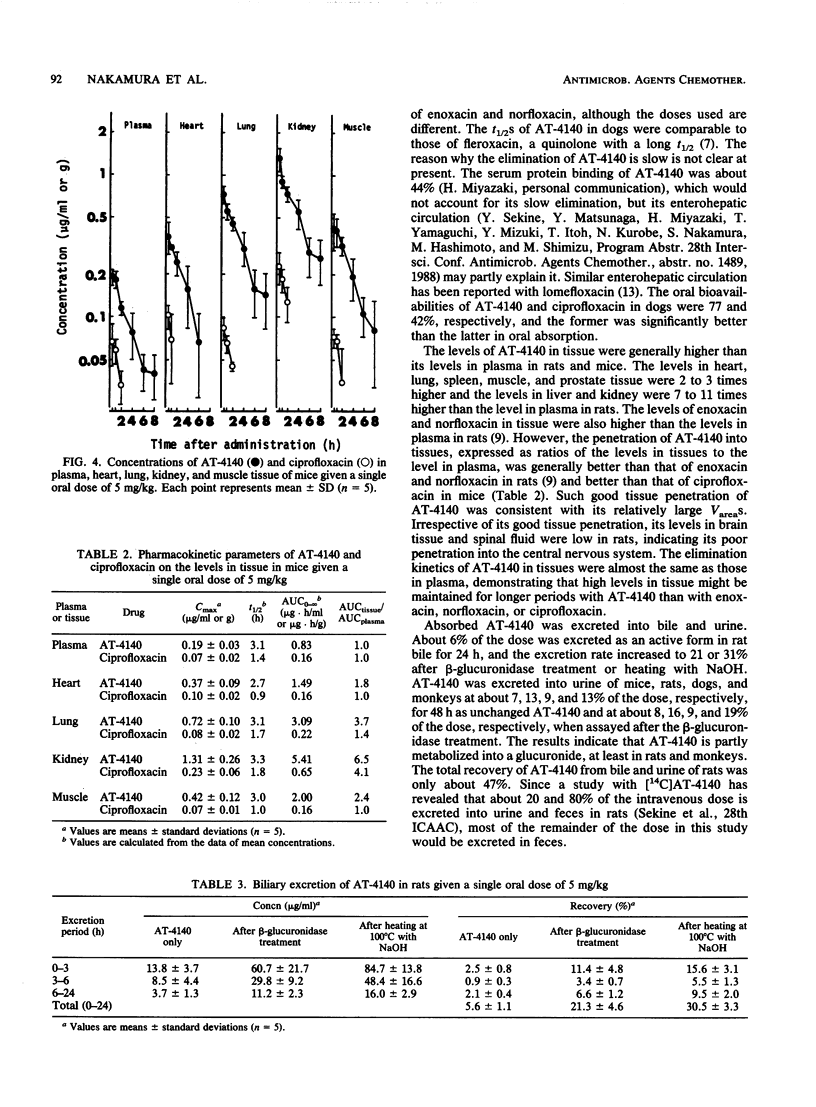
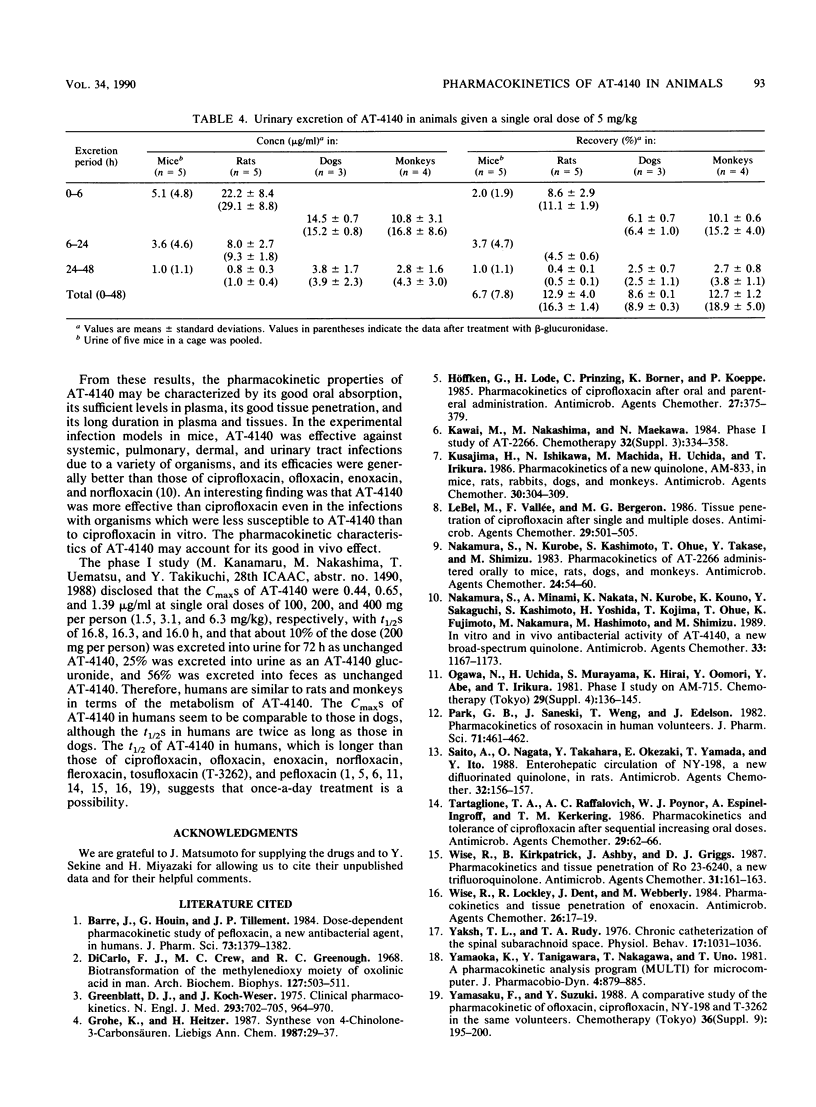
The pharmacokinetics of 5-amino-1-cyclopropyl-6,8-difluoro-1,4-dihydro-7-(cis-3,5-dimethyl-1- piperazinyl)-4-oxoquinoline-3-carboxylic acid (AT-4140) in experimental animals given a single oral dose of 5 mg/kg were studied. The mean peak levels of AT-4140 in plasma of mice, rats, dogs, and monkeys were 0.25, 0.50, 1.14, and 0.49 micrograms/ml, respectively, with mean elimination half-lives of 5.0, 3.8, 8.0, and 11.7 h, respectively. The oral bioavailability of AT-4140 calculated from the ratio of the areas under the concentration-time curve after oral and intravenous administration was 77% in dogs. The levels of AT-4140 in tissue in mice and rats were 1 to 11 times higher than the levels in plasma and 4 to 9 times higher than those of ciprofloxacin in mice. The mean 24-h biliary recovery of AT-4140 in rats was 5.6% of the dose and became 21.3% after beta-glucuronidase treatment. The mean 48-h urinary recoveries of AT-4140 in mice, rats, dogs, and monkeys were 6.7, 12.9, 8.6, and 12.7%, respectively, of the dose and were 7.8, 16.3, 8.9, and 18.9%, respectively, after beta-glucuronidase treatment. The pharmacokinetics of AT-4140 may be characterized by its good tissue penetration and its long half-life in plasma and tissues.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barre J., Houin G., Tillement J. P. Dose-dependent pharmacokinetic study of pefloxacin, a new antibacterial agent, in humans. J Pharm Sci. 1984 Oct;73(10):1379–1382. doi: 10.1002/jps.2600731014. [DOI] [PubMed] [Google Scholar]
- DiCarlo F. J., Crew M. C., Greenough R. C. Biotransformation of the methylenedioxy moeity of oxolinic acid in man. Arch Biochem Biophys. 1968 Sep 20;127(1):503–511. doi: 10.1016/0003-9861(68)90255-5. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Höffken G., Lode H., Prinzing C., Borner K., Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985 Mar;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusajima H., Ishikawa N., Machida M., Uchida H., Irikura T. Pharmacokinetics of a new quinolone, AM-833, in mice, rats, rabbits, dogs, and monkeys. Antimicrob Agents Chemother. 1986 Aug;30(2):304–309. doi: 10.1128/aac.30.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel M., Vallée F., Bergeron M. G. Tissue penetration of ciprofloxacin after single and multiple doses. Antimicrob Agents Chemother. 1986 Mar;29(3):501–505. doi: 10.1128/aac.29.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kurobe N., Kashimoto S., Ohue T., Takase Y., Shimizu M. Pharmacokinetics of AT-2266 administered orally to mice, rats, dogs, and monkeys. Antimicrob Agents Chemother. 1983 Jul;24(1):54–60. doi: 10.1128/aac.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Nakata K., Kurobe N., Kouno K., Sakaguchi Y., Kashimoto S., Yoshida H., Kojima T., Ohue T. In vitro and in vivo antibacterial activities of AT-4140, a new broad-spectrum quinolone. Antimicrob Agents Chemother. 1989 Aug;33(8):1167–1173. doi: 10.1128/aac.33.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G. B., Saneski J., Weng T., Edelson J. Pharmacokinetics of rosoxacin in human volunteers. J Pharm Sci. 1982 Apr;71(4):461–462. doi: 10.1002/jps.2600710424. [DOI] [PubMed] [Google Scholar]
- Saito A., Nagata O., Takahara Y., Okezaki E., Yamada T., Ito Y. Enterohepatic circulation of NY-198, a new difluorinated quinolone, in rats. Antimicrob Agents Chemother. 1988 Jan;32(1):156–157. doi: 10.1128/aac.32.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglione T. A., Raffalovich A. C., Poynor W. J., Espinel-Ingroff A., Kerkering T. M. Pharmacokinetics and tolerance of ciprofloxacin after sequential increasing oral doses. Antimicrob Agents Chemother. 1986 Jan;29(1):62–66. doi: 10.1128/aac.29.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Kirkpatrick B., Ashby J., Griggs D. J. Pharmacokinetics and tissue penetration of Ro 23-6240, a new trifluoroquinolone. Antimicrob Agents Chemother. 1987 Feb;31(2):161–163. doi: 10.1128/aac.31.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R., Dent J., Webberly M. Pharmacokinetics and tissue penetration of enoxacin. Antimicrob Agents Chemother. 1984 Jul;26(1):17–19. doi: 10.1128/aac.26.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh T. L., Rudy T. A. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976 Dec;17(6):1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]


