Abstract
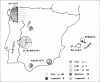
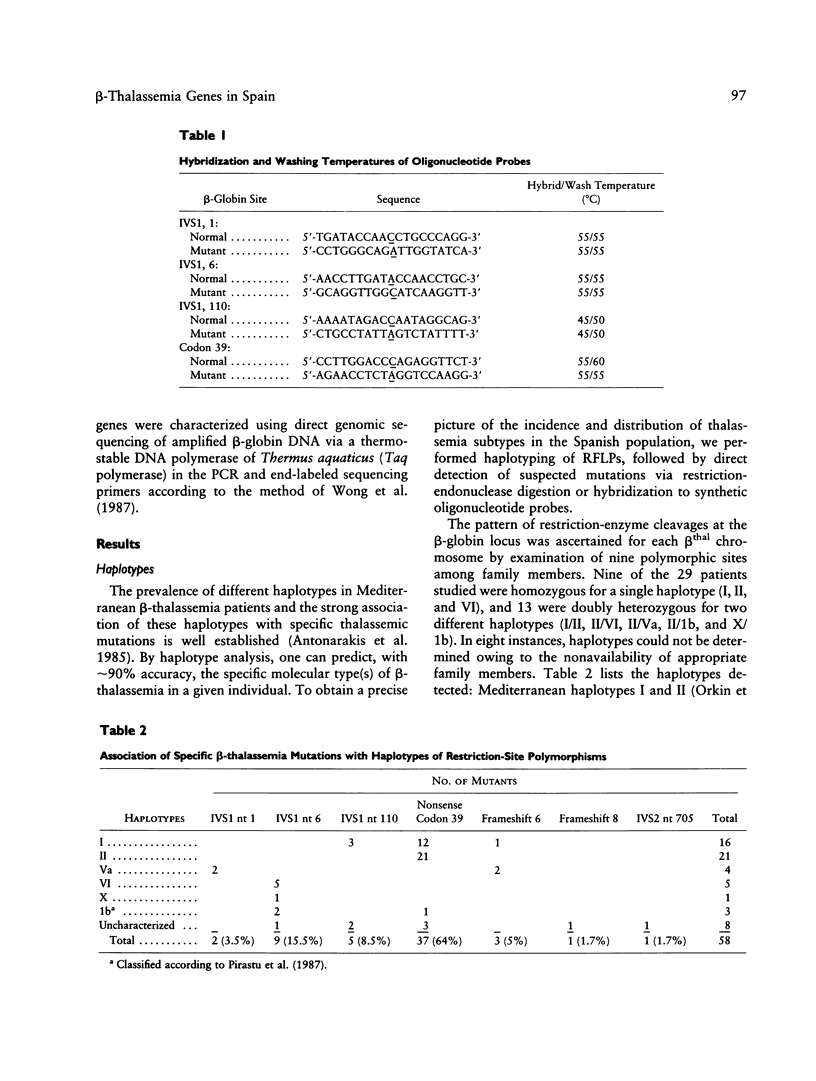
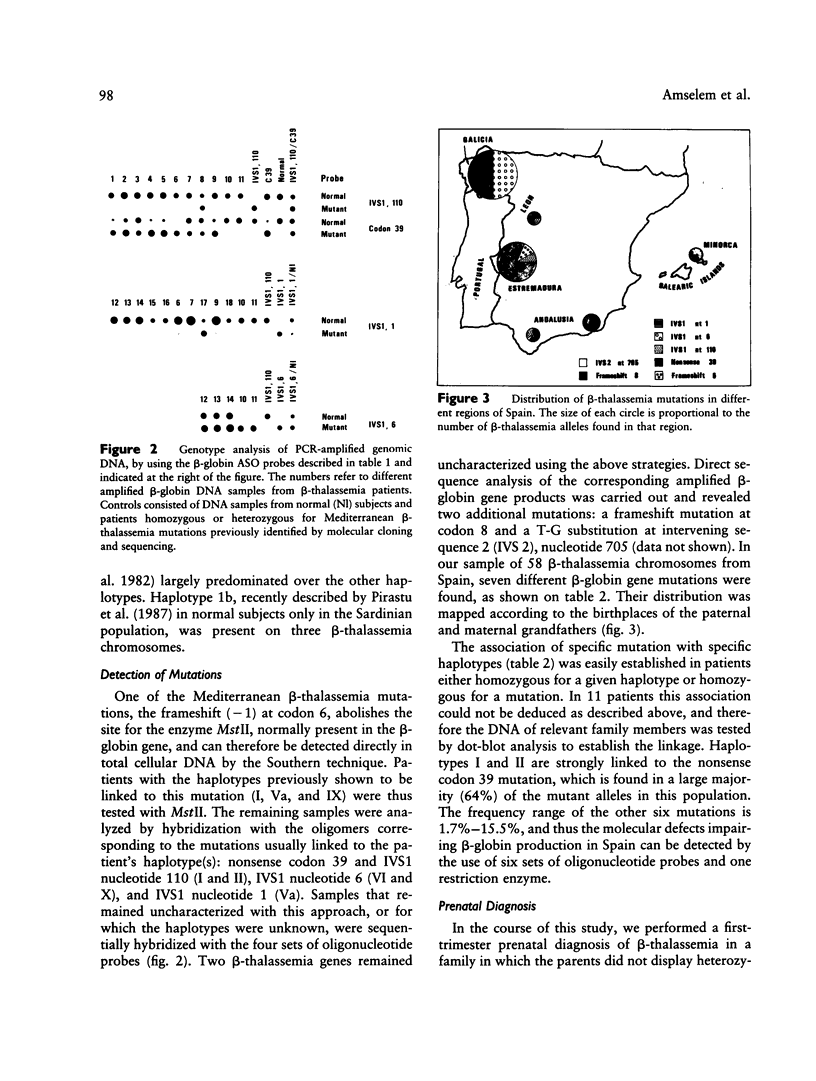
We have delineated the molecular lesions causing beta-thalassemia in Spain, a country that has witnessed the passage of different Mediterranean populations over the centuries, in order to evaluate the extent of heterogeneity of these mutations and to make possible simplified prenatal diagnosis of the disorder in that country. The use of the polymerase chain-reaction (PCR) technique to preferentially amplify beta-globin DNA sequences that contain the most frequent beta-thalassemia mutations in Mediterraneans enabled us to rapidly analyze 58 beta-thalassemia alleles in a dot-blot format either by hybridization with allele-specific radiolabeled oligonucleotide probes or by direct sequence analysis of the amplification product. The Spanish population carries seven different beta-thalassemia mutations; the nonsense codon 39 is predominant (64%), whereas the IVS1 position 110 mutation, the most common cause of beta-thalassemia in the eastern part of the Mediterranean basin, is underrepresented (8.5%). The IVS1 mutation at position 6 accounts for 15% of the defects and leads to a more severe form of beta+-thalassemia than originally described in most of the patients we studied. In this study, we demonstrate further the usefulness of the dot-blot hybridization of PCR-amplified genomic DNA in both rapid population surveys and prenatal diagnosis of beta-thalassemia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Kazazian H. H., Jr, Orkin S. H. DNA polymorphism and molecular pathology of the human globin gene clusters. Hum Genet. 1985;69(1):1–14. doi: 10.1007/BF00295521. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Alberti A., Kan Y. W. A beta-thalassemia lesion abolishes the same Mst II site as the sickle mutation. Nucleic Acids Res. 1983 Nov 25;11(22):7789–7794. doi: 10.1093/nar/11.22.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab F. F., Der Kaloustian V., Khouri F. P., Deeb S. S., Kan Y. W. The molecular basis of beta-thalassemia in Lebanon: application to prenatal diagnosis. Blood. 1987 Apr;69(4):1141–1145. [PubMed] [Google Scholar]
- Chibani J., Vidaud M., Duquesnoy P., Bergé-Lefranc J. L., Pirastu M., Ellouze F., Rosa J., Goossens M. The peculiar spectrum of beta-thalassemia genes in Tunisia. Hum Genet. 1988 Feb;78(2):190–192. doi: 10.1007/BF00278196. [DOI] [PubMed] [Google Scholar]
- Gomes M. P., da Costa M. G., Braga L. B., Cordeiro-Ferreira N. T., Loi A., Pirastu M., Cao A. Beta-thalassemia mutations in the Portuguese population. Hum Genet. 1988 Jan;78(1):13–15. doi: 10.1007/BF00291226. [DOI] [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Gorski J., Fiori M., Mach B. A new nonsense mutation as the molecular basis for beta thalassaemia. J Mol Biol. 1982 Jan 25;154(3):537–540. doi: 10.1016/s0022-2836(82)80012-0. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Lee K. Y., Furbetta M., Angius A., Cao A. Polymorphism of DNA sequence in the beta-globin gene region. Application to prenatal diagnosis of beta 0 thalassemia in Sardinia. N Engl J Med. 1980 Jan 24;302(4):185–188. doi: 10.1056/NEJM198001243020401. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Pergolizzi R., Spritz R. A., Spence S., Goossens M., Kan Y. W., Bank A. Two cloned beta thalassemia genes are associated with amber mutations at codon 39. Nucleic Acids Res. 1981 Dec 21;9(24):7065–7072. doi: 10.1093/nar/9.24.7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirastu M., Galanello R., Doherty M. A., Tuveri T., Cao A., Kan Y. W. The same beta-globin gene mutation is present on nine different beta-thalassemia chromosomes in a Sardinian population. Proc Natl Acad Sci U S A. 1987 May;84(9):2882–2885. doi: 10.1073/pnas.84.9.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnini G. P., Lopes M. C., Castanheira M. E., Wainscoat J. S., Wood W. G. Beta + thalassemia--Portuguese type: clinical, haematological and molecular studies of a newly defined form of beta thalassaemia. Br J Haematol. 1983 Jun;54(2):189–200. doi: 10.1111/j.1365-2141.1983.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Trecartin R. F., Liebhaber S. A., Chang J. C., Lee K. Y., Kan Y. W., Furbetta M., Angius A., Cao A. beta zero thalassemia in Sardinia is caused by a nonsense mutation. J Clin Invest. 1981 Oct;68(4):1012–1017. doi: 10.1172/JCI110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainscoat J. S., Old J. M., Weatherall D. J., Orkin S. H. The molecular basis for the clinical diversity of beta thalassaemia in Cypriots. Lancet. 1983 Jun 4;1(8336):1235–1237. doi: 10.1016/s0140-6736(83)92694-6. [DOI] [PubMed] [Google Scholar]
- Westaway D., Williamson R. An intron nucleotide sequence variant in a cloned beta +-thalassaemia globin gene. Nucleic Acids Res. 1981 Apr 24;9(8):1777–1788. doi: 10.1093/nar/9.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]