Abstract
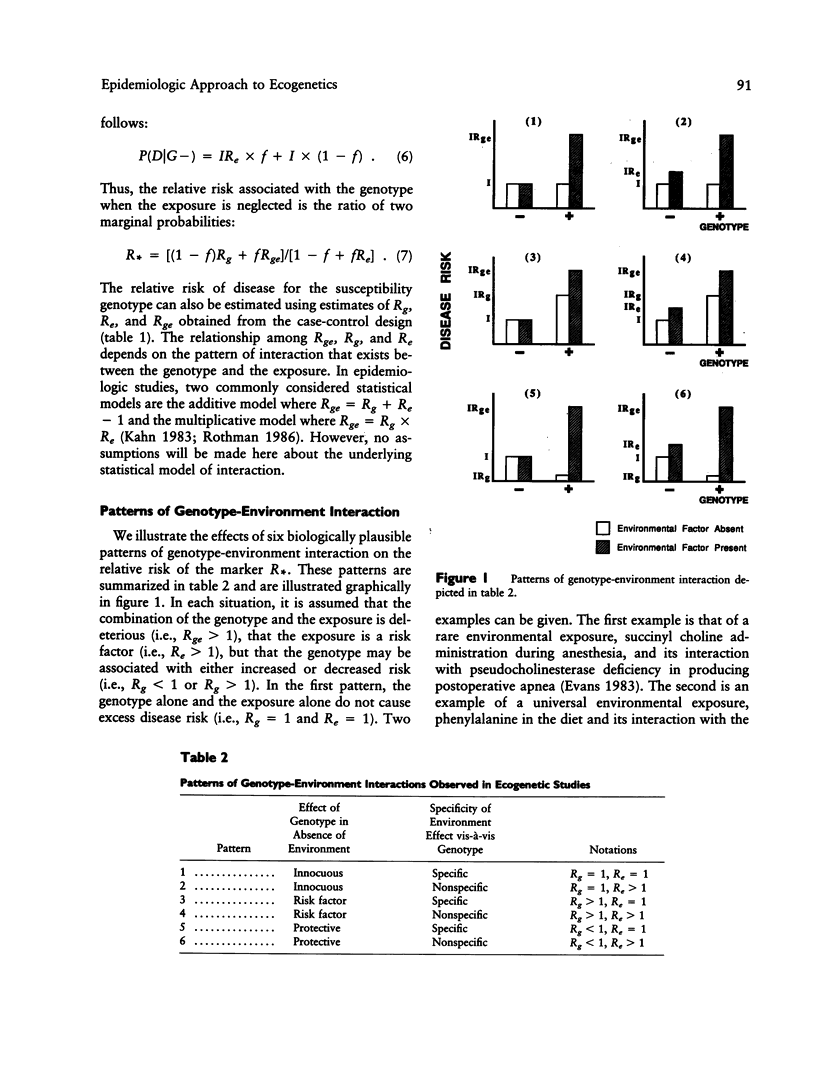
Although "ecogenetics" seeks to examine genetically mediated differences in susceptibility to environmental agents, researchers often examine the relation between genetic markers and disease without regard to environmental determinants. By using epidemiologic definitions of genotype-environment interaction, it can be shown that the relative risk of disease for the genetic marker is a function of the frequency of exposure to the environmental agent, the strength of interaction between the genotype and the agent, and the specificity of the environmental effect vis-à-vis the genotype. Using examples from the literature, we illustrate under six patterns of genotype-environment interaction that the relative risk associated with the marker can fluctuate markedly. However, with infrequent exposures, the relative risk is close to unity (implying no genetic effect) even in the face of strong genotype-environment interaction. Alternatively, elevated relative risks imply a frequent environmental exposure or a strong pattern of interaction. We suggest that genetic marker-disease associations be evaluated within the context of an epidemiologic study design that considers specific environmental determinants of risk.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayesh R., Idle J. R., Ritchie J. C., Crothers M. J., Hetzel M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984 Nov 8;312(5990):169–170. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- Barbeau A., Cloutier T., Roy M., Plasse L., Paris S., Poirier J. Ecogenetics of Parkinson's disease: 4-hydroxylation of debrisoquine. Lancet. 1985 Nov 30;2(8466):1213–1216. doi: 10.1016/s0140-6736(85)90743-3. [DOI] [PubMed] [Google Scholar]
- Cartwright R. A., Glashan R. W., Rogers H. J., Ahmad R. A., Barham-Hall D., Higgins E., Kahn M. A. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982 Oct 16;2(8303):842–845. doi: 10.1016/s0140-6736(82)90810-8. [DOI] [PubMed] [Google Scholar]
- Evans D. A. Survey of the human acetylator polymorphism in spontaneous disorders. J Med Genet. 1984 Aug;21(4):243–253. doi: 10.1136/jmg.21.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedde H. W. Okogenetik. Fortschr Med. 1979 Jan 25;97(4):127-8, 165-7. [PubMed] [Google Scholar]
- Khoury M. J., Stewart W., Beaty T. H. The effect of genetic susceptibility on causal inference in epidemiologic studies. Am J Epidemiol. 1987 Oct;126(4):561–567. doi: 10.1093/oxfordjournals.aje.a114695. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Nwachuku-Jarrett E. S., Reddy S. Increased sickling of parasitised erythrocytes as mechanism of resistance against malaria in the sickle-cell trait. Lancet. 1970 Feb 14;1(7642):319–321. doi: 10.1016/s0140-6736(70)90700-2. [DOI] [PubMed] [Google Scholar]
- Motulsky A. G. Pharmacogenetics and ecogenetics: the problem and its scope. Hum Genet Suppl. 1978;(1):1–3. [PubMed] [Google Scholar]
- Sing C. F., Boerwinkle E., Moll P. P. Strategies for elucidating the phenotypic and genetic heterogeneity of a chronic disease with a complex etiology. Prog Clin Biol Res. 1985;194:39–66. [PubMed] [Google Scholar]
- Vesell E. S. Advances in pharmacogenetics. Prog Med Genet. 1973;9:291–367. [PubMed] [Google Scholar]
- Ward R. H. Isolates in transition: a research paradigm for genetic epidemiology. Prog Clin Biol Res. 1985;194:147–177. [PubMed] [Google Scholar]


