Abstract
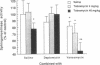
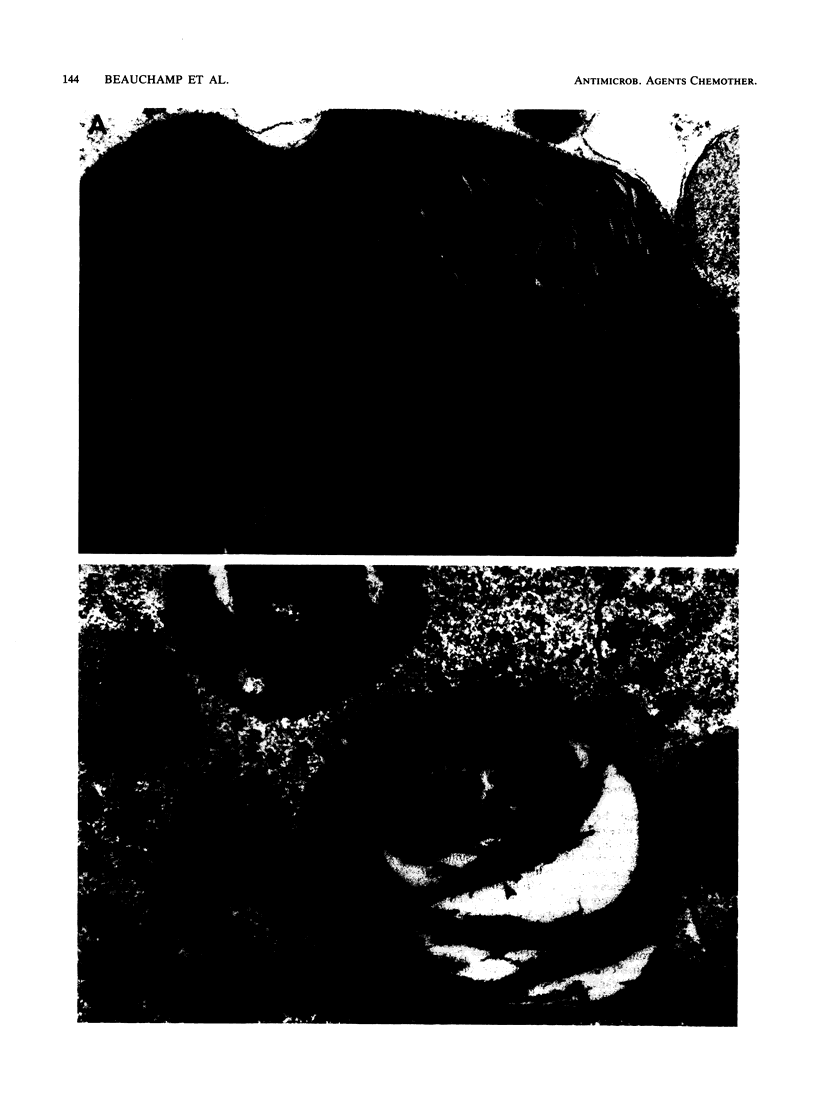
Daptomycin is a new biosynthetic antibiotic which belongs to a new class of drugs known as lipopeptides. The objective of this study was to evaluate the effects of daptomycin and vancomycin on tobramycin-induced nephrotoxicity. Female Sprague-Dawley rats were treated during 4 and 10 days with either saline (NaCl, 0.9%) or tobramycin at doses of 4 and 40 mg/kg per day (given every 12 h [q12h] intraperitoneally). Each treatment was combined with saline, daptomycin at a dose of 20 mg/kg per day (given q12h subcutaneously), and ancomycin at a dose of 50 mg/kg per day (given q12h subcutaneously). Daptomycin and vancomycin had no effect on the intracortical accumulation of tobramycin. Daptomycin did not accumulate in renal tissue even after 10 days of treatment. Tobramycin given at a dose of 40 mg/kg per day during 10 days induced a significant inhibition of sphingomyelinase activity in the renal cortex (P less than 0.01) and increased cellular regeneration (P less than 0.01), as measured by the incorporation of [3H]thymidine into DNA of the renal cortex. These changes were minimal when daptomycin was combined with tobramycin. Histologically, signs of tobramycin toxicity were also less severe in the presence of daptomycin. The intracortical accumulation of vancomycin was not modified by tobramycin. The sphingomyelinase activity was significantly more inhibited (P less than 0.01) when vancomycin was associated with tobramycin (4 and 40 mg/kg) without affecting the rate of [3H]thymidine incorporation into DNA. Histologically, signs of tobramycin toxicity were not affected by vancomuycin, but the cellular vacuolizations which were also observed in vancomycin-treated animals were still present in the proximal tubular cells of animals that were treated with the combination vancomycin-tobramycin. This study strongly suggests that daptomycin protects animals from tobramycin-induced nephrotoxicity but that vancomycin may enhance the effect of tobramycin. We conclude that daptomycin is safe and protects kidney cells from tobramycin-induced nephrotoxicity.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Hobbs J. N., Alborn W. E., Jr Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob Agents Chemother. 1987 Jul;31(7):1093–1099. doi: 10.1128/aac.31.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert-Tulkens G., Van Hoof F., Tulkens P. Gentamicin-induced lysosomal phospholipidosis in cultured rat fibroblasts. Quantitative ultrastructural and biochemical study. Lab Invest. 1979 Apr;40(4):481–491. [PubMed] [Google Scholar]
- Beauchamp D., Laurent G., Maldague P., Tulkens P. M. Reduction of gentamicin nephrotoxicity by the concomitant administration of poly-l-aspartic acid and poly-l-asparagine in rats. Arch Toxicol Suppl. 1986;9:306–309. doi: 10.1007/978-3-642-71248-7_52. [DOI] [PubMed] [Google Scholar]
- Bennett W. M., Elliott W. C., Houghton D. C., Gilbert D. N., DeFehr J., McCarron D. A. Reduction of experimental gentamicin nephrotoxicity in rats by dietary calcium loading. Antimicrob Agents Chemother. 1982 Sep;22(3):508–512. doi: 10.1128/aac.22.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C. A., Beaudette F., Trenholm G. Comparative in-vitro activity of LY146032 a new peptolide, with vancomycin and eight other agents against gram-positive organisms. J Antimicrob Chemother. 1987 Aug;20(2):191–196. doi: 10.1093/jac/20.2.191. [DOI] [PubMed] [Google Scholar]
- Bergeron M. G., Marois Y., Kuehn C., Silverblatt F. J. Autoradiographic study of tobramycin uptake by proximal and distal tubules of normal and pyelonephritic rats. Antimicrob Agents Chemother. 1987 Sep;31(9):1359–1364. doi: 10.1128/aac.31.9.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush L. M., Boscia J. A., Kaye D. Daptomycin (LY146032) treatment of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1988 Jun;32(6):877–881. doi: 10.1128/aac.32.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Willey S., Reiszner E., Spitzer P. G., Caputo G., Moellering R. C., Jr In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986 Oct;30(4):532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English J., Gilbert D. N., Kohlhepp S., Kohnen P. W., Mayor G., Houghton D. C., Bennett W. M. Attenuation of experimental tobramycin nephrotoxicity by ticarcillin. Antimicrob Agents Chemother. 1985 Jun;27(6):897–902. doi: 10.1128/aac.27.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Moellering R. C., Jr Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother. 1983 Jan;23(1):138–141. doi: 10.1128/aac.23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N., Wood C. A., Kohlhepp S. J., Kohnen P. W., Houghton D. C., Finkbeiner H. C., Lindsley J., Bennett W. M. Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J Infect Dis. 1989 May;159(5):945–953. doi: 10.1093/infdis/159.5.945. [DOI] [PubMed] [Google Scholar]
- Giurgea-Marion L., Toubeau G., Laurent G., Heuson-Stiennon J. A., Tulkens P. M. Impairment of lysosome-pinocytic vesicle fusion in rat kidney proximal tubules after treatment with gentamicin at low doses. Toxicol Appl Pharmacol. 1986 Nov;86(2):271–285. doi: 10.1016/0041-008x(86)90058-x. [DOI] [PubMed] [Google Scholar]
- Humes H. D., Sastrasinh M., Weinberg J. M. Calcium is a competitive inhibitor of gentamicin-renal membrane binding interactions and dietary calcium supplementation protects against gentamicin nephrotoxicity. J Clin Invest. 1984 Jan;73(1):134–147. doi: 10.1172/JCI111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M., Erdmann G., Habermann E. The renal handling of polybasic drugs. 1. Gentamicin and aprotinin in intact animals. Naunyn Schmiedebergs Arch Pharmacol. 1977 Oct;300(1):57–66. doi: 10.1007/BF00505080. [DOI] [PubMed] [Google Scholar]
- Kacew S. Inhibition of gentamicin-induced nephrotoxicity by pyridoxal-5'-phosphate in the rat. J Pharmacol Exp Ther. 1989 Jan;248(1):360–366. [PubMed] [Google Scholar]
- Kosek J. C., Mazze R. I., Cousins M. J. Nephrotoxicity of gentamicin. Lab Invest. 1974 Jan;30(1):48–57. [PubMed] [Google Scholar]
- Laurent G., Carlier M. B., Rollman B., Van Hoof F., Tulkens P. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem Pharmacol. 1982 Dec 1;31(23):3861–3870. doi: 10.1016/0006-2952(82)90303-3. [DOI] [PubMed] [Google Scholar]
- Laurent G., Maldague P., Carlier M. B., Tulkens P. M. Increased renal DNA synthesis in vivo after administration of low doses of gentamicin to rats. Antimicrob Agents Chemother. 1983 Oct;24(4):586–593. doi: 10.1128/aac.24.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. M., Michael U. F. The protective effect of nitrendipine on gentamicin acute renal failure in rats. Exp Mol Pathol. 1985 Aug;43(1):107–114. doi: 10.1016/0014-4800(85)90060-7. [DOI] [PubMed] [Google Scholar]
- MAUNSBACH A. B., MADDEN S. C., LATTA H. Light and electron microscopic changes in proximal tubules of rats after administration of glucose, mannitol, sucrose, or dextran. Lab Invest. 1962 Jun;11:421–432. [PubMed] [Google Scholar]
- Marre R., Schulz E., Anders T., Sack K. Renal tolerance and pharmacokinetics of vancomycin in rats. J Antimicrob Chemother. 1984 Sep;14(3):253–260. doi: 10.1093/jac/14.3.253. [DOI] [PubMed] [Google Scholar]
- Marre R., Schulz E., Hedtke D., Sack K. Influence of fosfomycin and tobramycin on vancomycin-induced nephrotoxicity. Infection. 1985 Jul-Aug;13(4):190–192. doi: 10.1007/BF01642811. [DOI] [PubMed] [Google Scholar]
- Miniter P. M., Patterson T. F., Johnson M. A., Andriole V. T. Activity of LY146032 in vitro and in experimental enterococcal pyelonephritis. Antimicrob Agents Chemother. 1987 Aug;31(8):1199–1203. doi: 10.1128/aac.31.8.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J. P., Viotte G., Vandewalle A., Van Hoof F., Tulkens P., Fillastre J. P. Gentamicin-induced nephrotoxicity: a cell biology approach. Kidney Int. 1980 Nov;18(5):583–590. doi: 10.1038/ki.1980.176. [DOI] [PubMed] [Google Scholar]
- Nahata M. C. Lack of nephrotoxicity in pediatric patients receiving concurrent vancomycin and aminoglycoside therapy. Chemotherapy. 1987;33(4):302–304. doi: 10.1159/000238512. [DOI] [PubMed] [Google Scholar]
- Pohlod D. J., Saravolatz L. D., Somerville M. M. In-vitro susceptibility of gram-positive cocci to LY146032 teicoplanin, sodium fusidate, vancomycin, and rifampicin. J Antimicrob Chemother. 1987 Aug;20(2):197–202. doi: 10.1093/jac/20.2.197. [DOI] [PubMed] [Google Scholar]
- Sapico F. L., Ginunas V. J., Canawati H. N., Montgomerie J. Z. LY146032, alone and in combination with gentamicin, for the treatment of enterococcal pyelonephritis in the rat model. Antimicrob Agents Chemother. 1988 Jan;32(1):81–83. doi: 10.1128/aac.32.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastrasinh M., Knauss T. C., Weinberg J. M., Humes H. D. Identification of the aminoglycoside binding site in rat renal brush border membranes. J Pharmacol Exp Ther. 1982 Aug;222(2):350–358. [PubMed] [Google Scholar]
- Silverblatt F. J., Kuehn C. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int. 1979 Apr;15(4):335–345. doi: 10.1038/ki.1979.45. [DOI] [PubMed] [Google Scholar]
- Sorrell T. C., Collignon P. J. A prospective study of adverse reactions associated with vancomycin therapy. J Antimicrob Chemother. 1985 Aug;16(2):235–241. doi: 10.1093/jac/16.2.235. [DOI] [PubMed] [Google Scholar]
- Swinney V. R., Rudd C. C. Nephrotoxicity of vancomycin-gentamicin therapy in pediatric patients. J Pediatr. 1987 Mar;110(3):497–498. doi: 10.1016/s0022-3476(87)80533-4. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978 Feb 15;27(4):415–424. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- Williams P. D., Hottendorf G. H., Bennett D. B. Inhibition of renal membrane binding and nephrotoxicity of aminoglycosides. J Pharmacol Exp Ther. 1986 Jun;237(3):919–925. [PubMed] [Google Scholar]
- Wood C. A., Finkbeiner H. C., Kohlhepp S. J., Kohnen P. W., Gilbert D. N. Influence of daptomycin on staphylococcal abscesses and experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother. 1989 Aug;33(8):1280–1285. doi: 10.1128/aac.33.8.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. A., Kohlhepp S. J., Kohnen P. W., Houghton D. C., Gilbert D. N. Vancomycin enhancement of experimental tobramycin nephrotoxicity. Antimicrob Agents Chemother. 1986 Jul;30(1):20–24. doi: 10.1128/aac.30.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]