Abstract
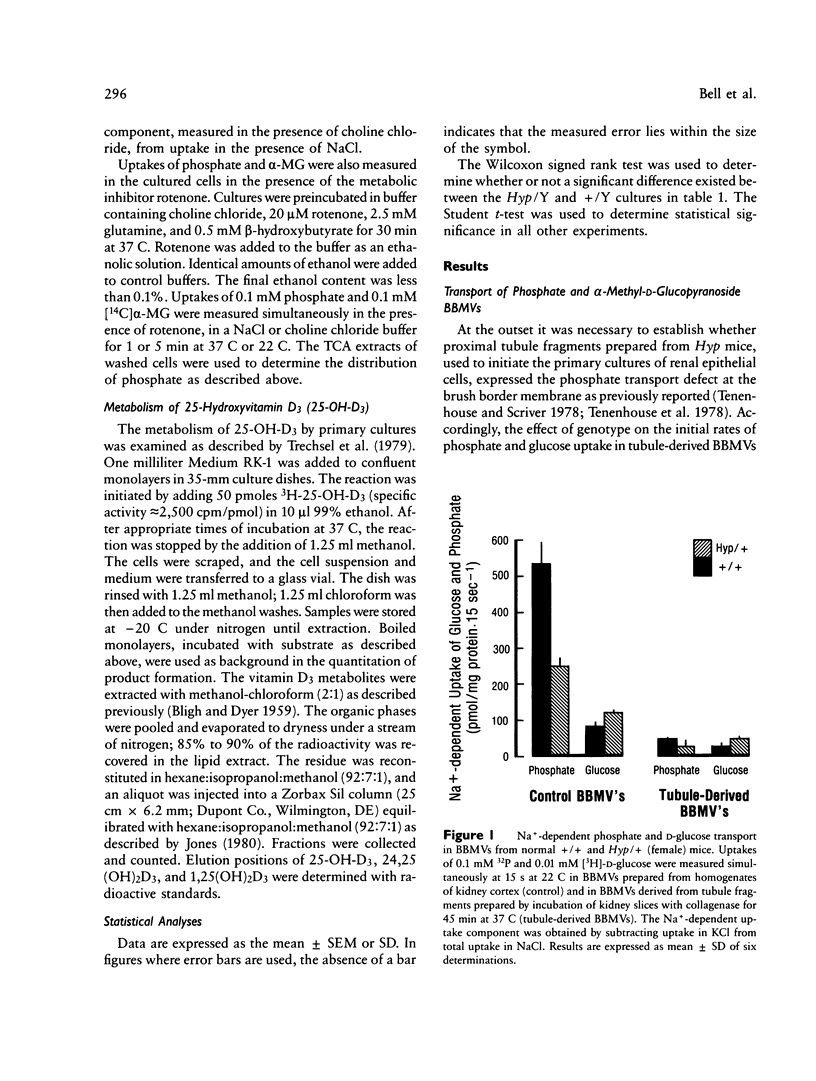
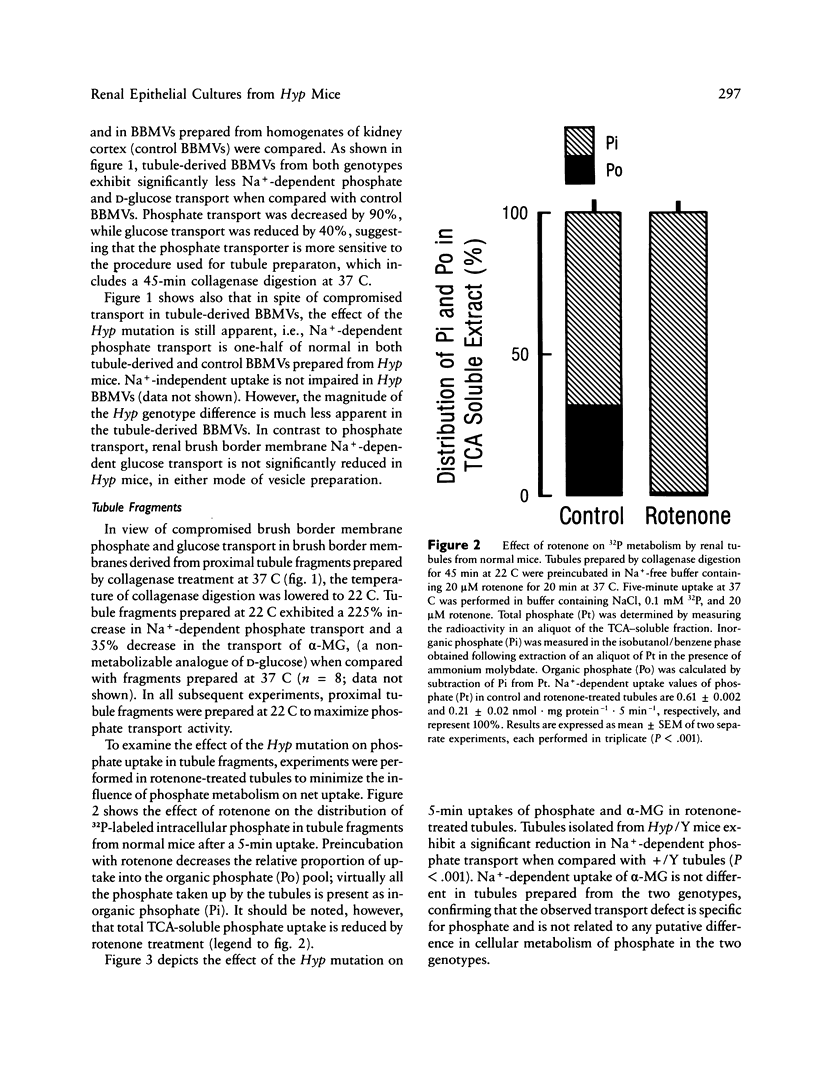
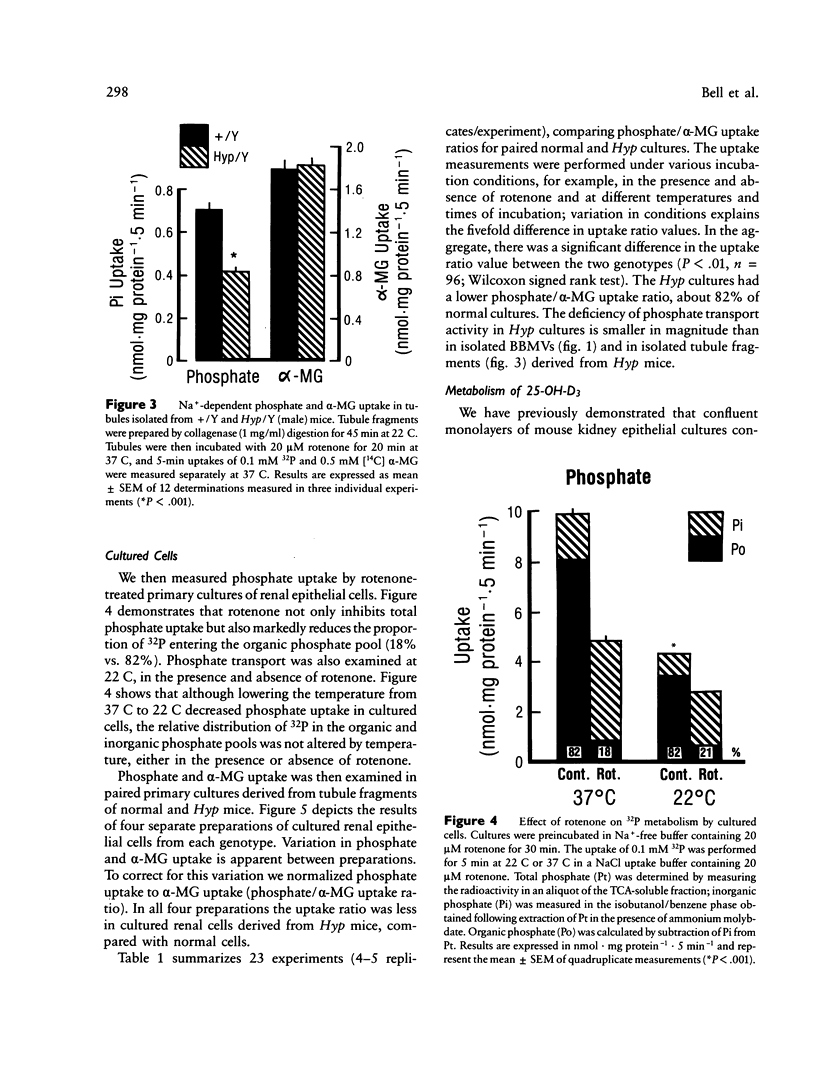
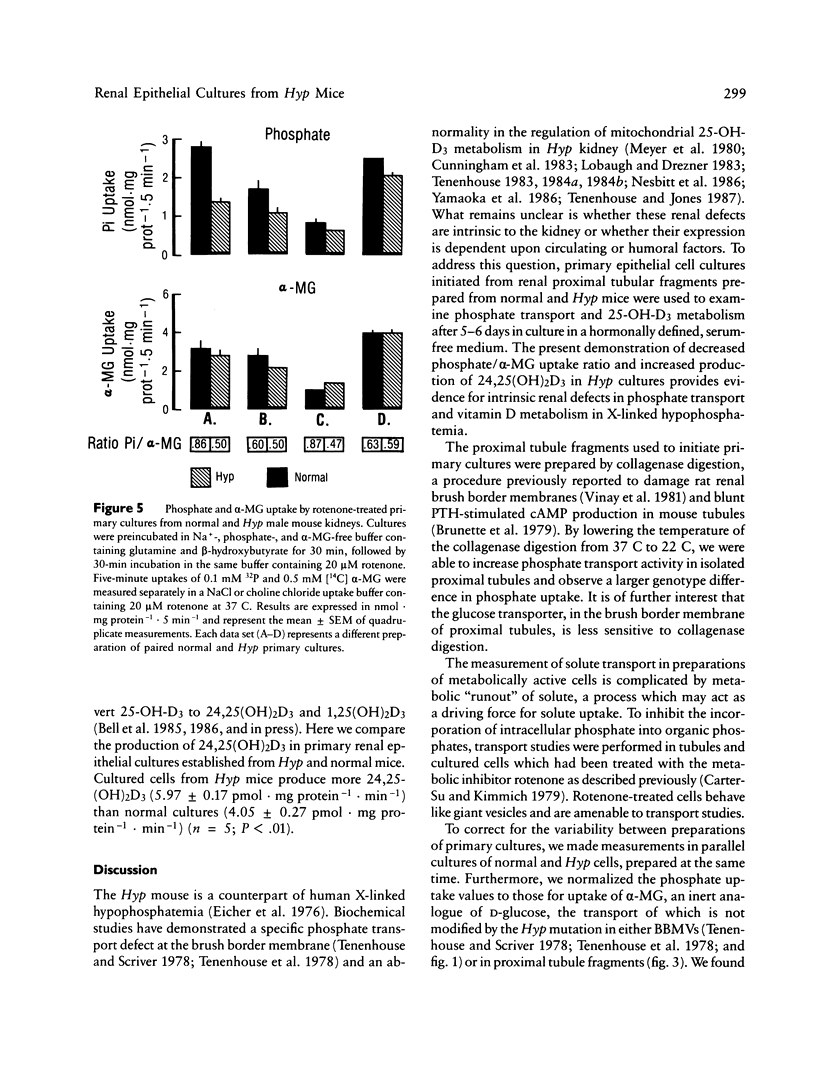
Mutation in a gene (symbol Hyp) on the X chromosome causes hypophosphatemia in the mouse. The murine phenotype is a counterpart of X-linked hypophosphatemia in man. Both exhibit impaired renal reabsorption of phosphate in vivo. In vitro studies in the Hyp mouse have shown decreased Na+-dependent phosphate transport at the brush border membrane and abnormal mitochondrial vitamin D metabolism. To determine whether the mutant renal phenotype is intrinsic to the kidney or dependent upon putative extrinsic humoral factor(s) for its expression, we established primary cultures of renal epithelial cells from normal and Hyp male mouse kidneys. The cells are derived from proximal tubule. Initial uptake rates of phosphate and alpha-methyl-D-glucopyranoside (alpha-MG), a metabolically inert analogue of D-glucose, were measured simultaneously in confluent monolayers exhibiting epithelial polarity and tight junctions. The mean phosphate/alpha-MG uptake ratio in Hyp cultures was 82% of that in normal cells (P less than 0.01, n = 96). Moreover, the production of 24,25-dihydroxyvitamin D3 was significantly elevated in confluent cultures of Hyp cells relative to normal cells. These results imply that the Hyp gene is expressed in situ in renal epithelium and suggest that humoral factors are not necessary for the mutant renal phenotype in X-linked hypophosphatemia of mouse and man.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S. Oncogenic hypophosphatemic osteomalacia. Kidney Int. 1983 Jul;24(1):113–123. doi: 10.1038/ki.1983.133. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell C. L., Tenenhouse H. S., Scriver C. R. Isolation and culture of murine renal proximal tubule cells: a system to study solute transport in mutants. Ann N Y Acad Sci. 1985;456:398–400. doi: 10.1111/j.1749-6632.1985.tb14890.x. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. Proteins of the kidney microvillus membrane. Identification of subunits after sodium dodecylsullphate/polyacrylamide-gel electrophoresis. Biochem J. 1976 Nov;159(2):395–407. doi: 10.1042/bj1590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette M. G., Chabardes D., Imbert-Teboul M., Clique A., Montégut M., Morel F. Hormone-sensitive adenylate cyclase along the nephron of genetically hypophosphatemic mice. Kidney Int. 1979 Apr;15(4):357–369. doi: 10.1038/ki.1979.47. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Kimmich G. A. Membrane potentials and sugar transport by ATP-depleted intestinal cells: effect of anion gradients. Am J Physiol. 1979 Jul;237(1):C67–C74. doi: 10.1152/ajpcell.1979.237.1.C64. [DOI] [PubMed] [Google Scholar]
- Chung S. D., Alavi N., Livingston D., Hiller S., Taub M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J Cell Biol. 1982 Oct;95(1):118–126. doi: 10.1083/jcb.95.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D. E., Koltay M., Scriver C. R. Sulfate transport by mouse renal cortical slices does not represent uptake by brush-border membrane. Biochim Biophys Acta. 1984 Sep 19;776(1):113–121. doi: 10.1016/0005-2736(84)90257-8. [DOI] [PubMed] [Google Scholar]
- Cowgill L. D., Goldfarb S., Lau K., Slatopolsky E., Agus Z. S. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest. 1979 Jun;63(6):1203–1210. doi: 10.1172/JCI109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J., Gomes H., Seino Y., Chase L. R. Abnormal 24-hydroxylation of 25-hydroxyvitamin D in the X-linked hypophosphatemic mouse. Endocrinology. 1983 Feb;112(2):633–638. doi: 10.1210/endo-112-2-633. [DOI] [PubMed] [Google Scholar]
- Eicher E. M., Southard J. L., Scriver C. R., Glorieux F. H. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukase M., Avioli L. V., Birge S. J., Chase L. R. Abnormal regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity by calcium and calcitonin in renal cortex from hypophosphatemic (Hyp) mice. Endocrinology. 1984 Apr;114(4):1203–1207. doi: 10.1210/endo-114-4-1203. [DOI] [PubMed] [Google Scholar]
- Giasson S. D., Brunette M. G., Danan G., Vigneault N., Carriere S. Micropuncture study of renal phosphorus transport in hypophosphatemic vitamin D resistant rickets mice. Pflugers Arch. 1977 Oct 19;371(1-2):33–38. doi: 10.1007/BF00580769. [DOI] [PubMed] [Google Scholar]
- Goldman H., Wong I., Patel Y. C. A study of the structural and biochemical development of human fetal islets of Langerhans. Diabetes. 1982 Oct;31(10):897–902. doi: 10.2337/diab.31.10.897. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Torikai S., Kurokawa K. Localization of 25-hydroxyvitamin D3 1 alpha-hydroxylase and 24-hydroxylase along the rat nephron. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1199–1203. doi: 10.1073/pnas.78.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebzak G. M., Roos B. A., Meyer R. A., Jr Secondary hyperparathyroidism in X-linked hypophosphatemic mice. Endocrinology. 1982 Aug;111(2):650–652. doi: 10.1210/endo-111-2-650. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Fukase M., Nakada M., Fujita T. Defective adaptation to a low phosphate environment by cultured renal tubular cells from X-linked hypophosphatemic (Hyp) mice. Biochem Biophys Res Commun. 1987 Apr 29;144(2):763–769. doi: 10.1016/s0006-291x(87)80030-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Gray R. W., Meyer M. H. Abnormal vitamin D metabolism in the X-linked hypophosphatemic mouse. Endocrinology. 1980 Nov;107(5):1577–1581. doi: 10.1210/endo-107-5-1577. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Drezner M. K., Lobaugh B. Abnormal parathyroid hormone stimulation of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the hypophosphatemic mouse. Evidence for a generalized defect of vitamin D metabolism. J Clin Invest. 1986 Jan;77(1):181–187. doi: 10.1172/JCI112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posillico J. T., Lobaugh B., Muhlbaier L. H., Drezner M. K. Abnormal parathyroid function in the X-linked hypophosphatemic mouse. Calcif Tissue Int. 1985 Jul;37(4):418–422. doi: 10.1007/BF02553712. [DOI] [PubMed] [Google Scholar]
- Taub N., Livingston D. The development of serum-free hormone-supplemented media for primary kidney cultures and their use in examining renal functions. Ann N Y Acad Sci. 1981;372:406–421. doi: 10.1111/j.1749-6632.1981.tb15491.x. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Jones G. Effect of the X-linked Hyp mutation and vitamin D status on induction of renal 25-hydroxyvitamin D3-24-hydroxylase. Endocrinology. 1987 Feb;120(2):609–616. doi: 10.1210/endo-120-2-609. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R., McInnes R. R., Glorieux F. H. Renal handling of phosphate in vivo and in vitro by the X-linked hypophosphatemic male mouse: evidence for a defect in the brush border membrane. Kidney Int. 1978 Sep;14(3):236–244. doi: 10.1038/ki.1978.115. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. The defect in transcellular transport of phosphate in the nephron is located in brush-border membranes in X-linked hypophosphatemia (Hyp mouse model). Can J Biochem. 1978 Jun;56(6):640–646. doi: 10.1139/o78-096. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Bonjour J. P., Fleisch H. Regulation of the metabolism of 25-hydroxyvitamin D3 in primary cultures of chick kidney cells. J Clin Invest. 1979 Jul;64(1):206–217. doi: 10.1172/JCI109441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VESTERGAARD-BOGIND B. DETERMINATION ON A MICRO SCALE OF CONCENTRATION AND SPECIFIC RADIOACTIVITY OF INORGANIC PHOSPHATE IONS IN WHOLE BLOOD AND PACKED RED CELLS. Scand J Clin Lab Invest. 1964;16:457–464. doi: 10.3109/00365516409060539. [DOI] [PubMed] [Google Scholar]
- Vinay P., Gougoux A., Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981 Oct;241(4):F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Seino Y., Satomura K., Tanaka Y., Yabuuchi H., Haussler M. R. Abnormal relationship between serum phosphate concentration and renal 25-hydroxycholecalciferol-1-alpha-hydroxylase activity in X-linked hypophosphatemic mice. Miner Electrolyte Metab. 1986;12(3):194–198. [PubMed] [Google Scholar]


