Abstract
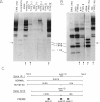
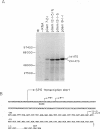
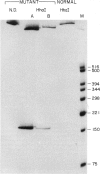
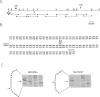
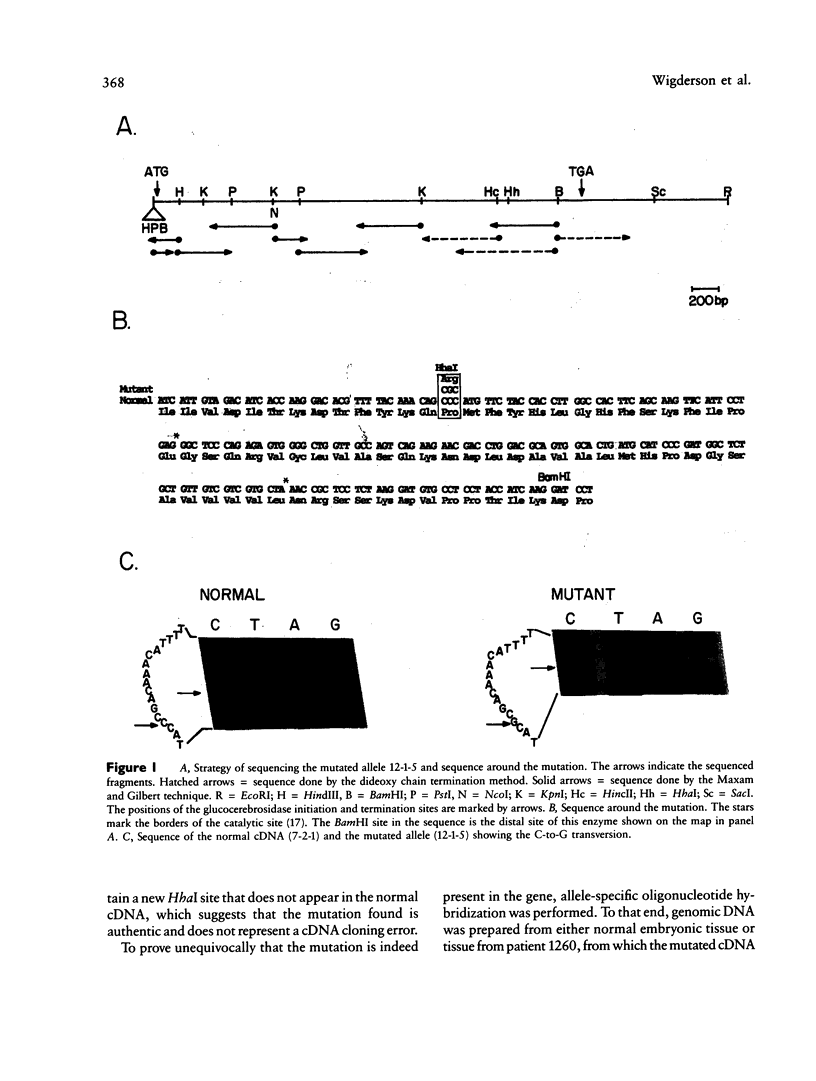
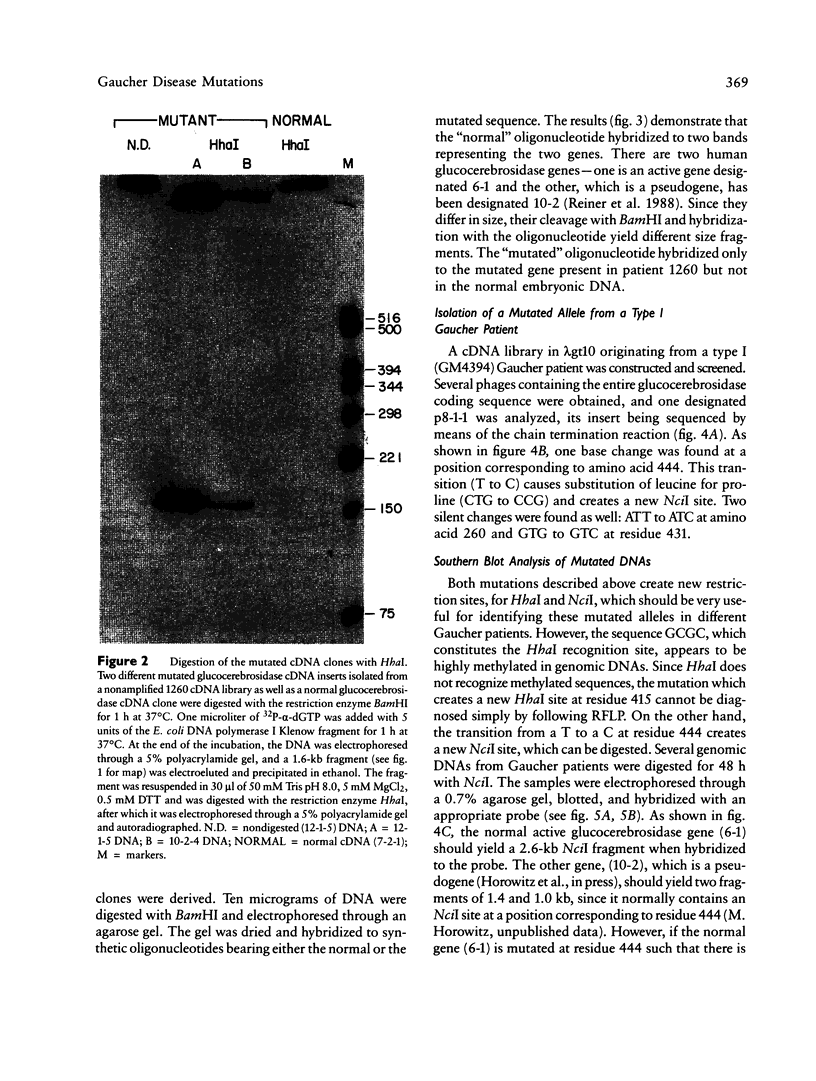
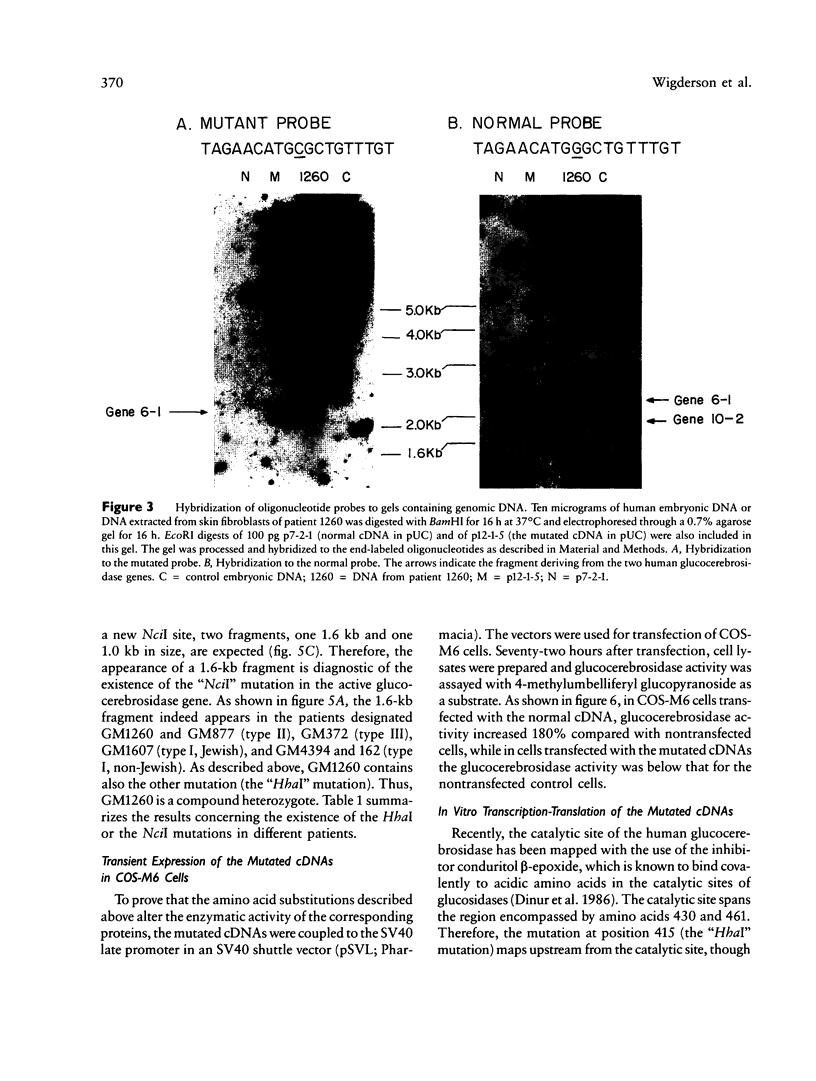
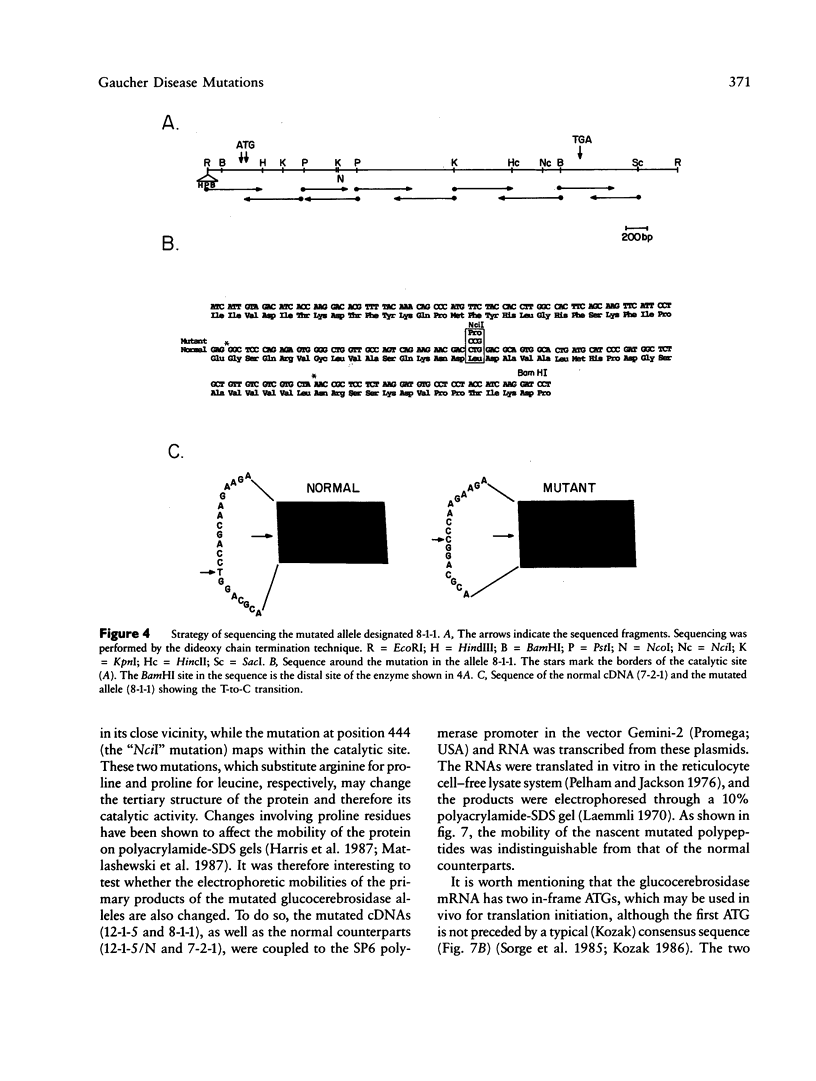
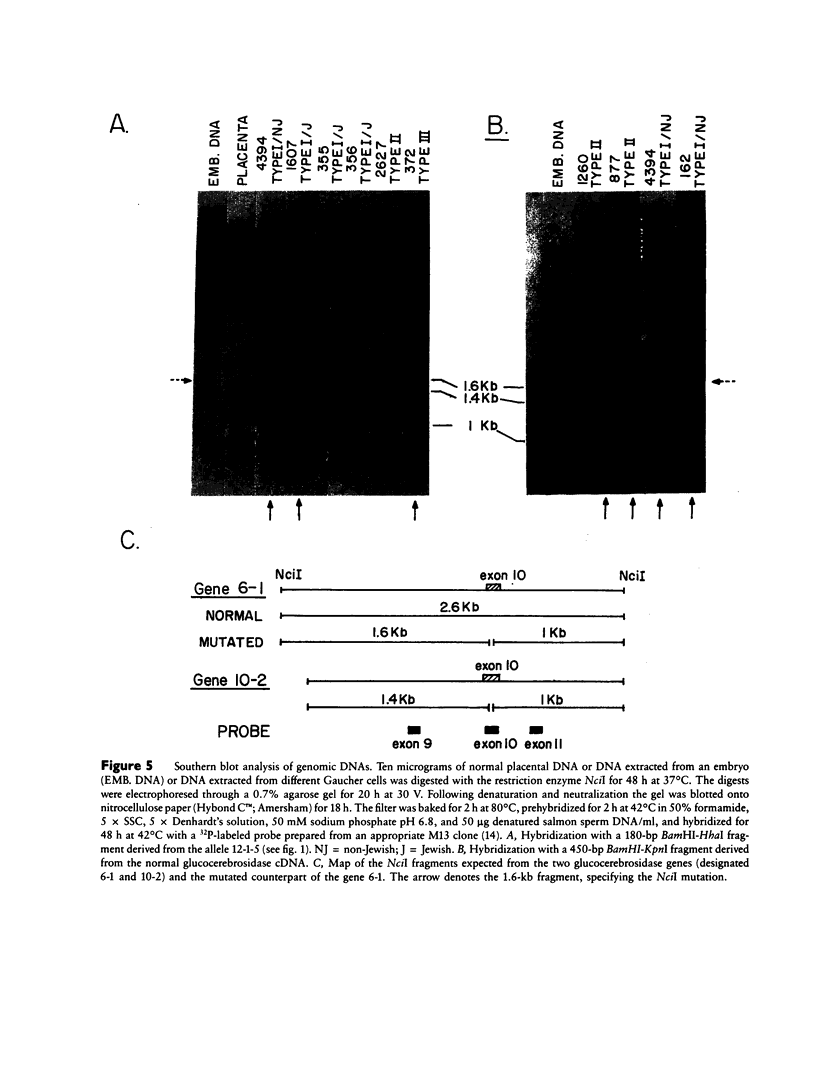
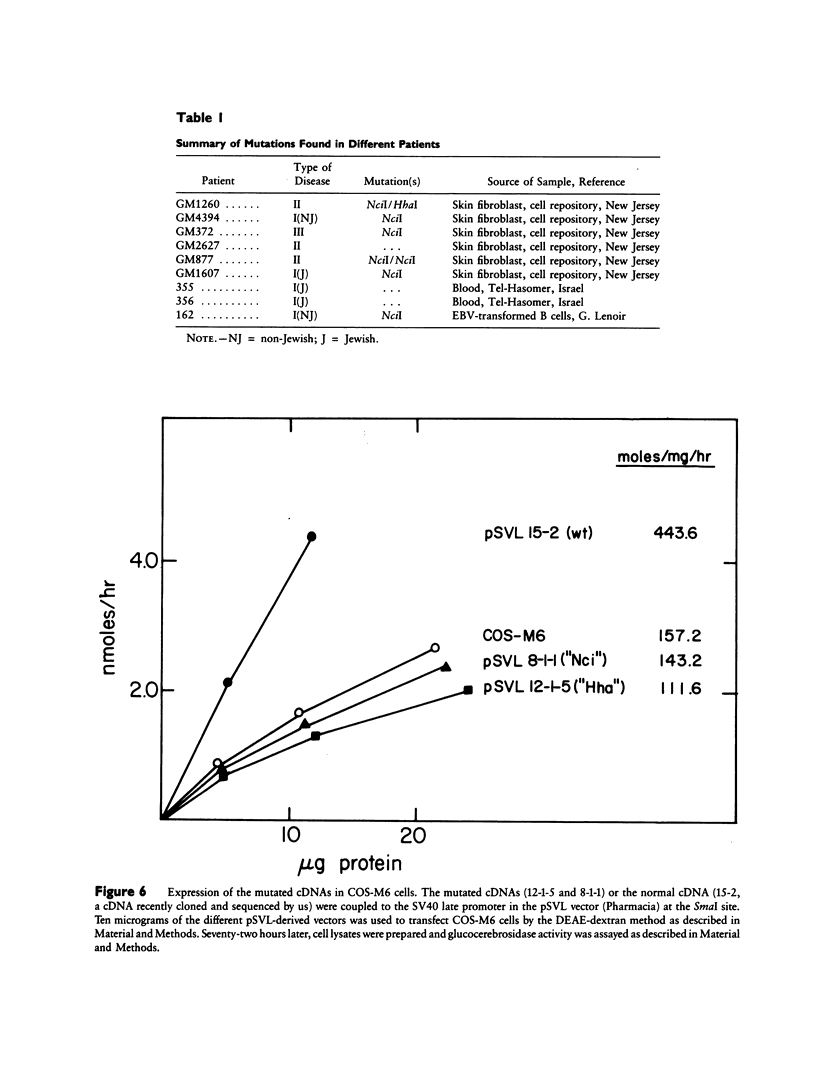
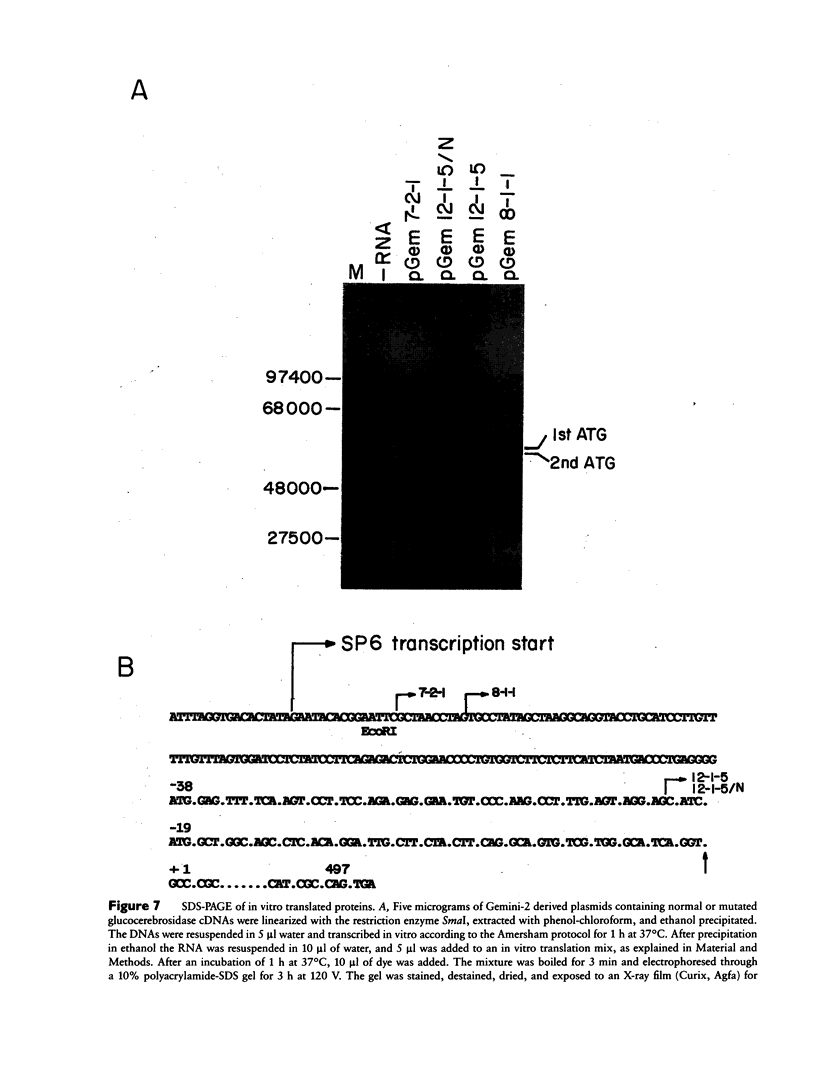
Mutated cDNA clones containing the entire coding sequence of human glucocerebrosidase were isolated from libraries originated from Gaucher patients. Sequence analysis of a mutated cDNA derived from a type II Gaucher patient revealed a C-to-G transversion causing a substitution of an arginine for a proline at residue 415. This change creates a new cleavage site for the enzyme HhaI in the mutated cDNA. Allele-specific oligonucleotide hybridization made it possible to show that this mutation exists in the genomic DNA of the patient. From a cDNA library originated from a type I Gaucher patient, a mutated allele was cloned that contains a T-to-C transition causing a substitution of proline for leucine at residue 444 and creating a new NciI site. This mutation is identical to that described by S. Tsuji and colleagues in genomic DNA from type I, type II, and type III patients. Since the new NciI site generates RFLP, it was used to test the existence of this mutated allele in several Gaucher patients by Southern blot analysis. This allele was found in type I (Jewish and non-Jewish), type II, and type III Gaucher patients. These findings led us to conclude that the patient suffering from type II disease (denoted GM1260) carried both mutations described above. Any one of the amino acid changes described reduces the glucocerebrosidase activity as tested by transfection of COS cells with expression vectors harboring the mutated cDNAs. The base changes in the two mutated cDNAs do not affect the electrophoretic mobility of the corresponding polypeptides on an SDS polyacrylamide gel.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY R. O., KANFER J. N., SHAPIRO D. METABOLISM OF GLUCOCEREBROSIDES. II. EVIDENCE OF AN ENZYMATIC DEFICIENCY IN GAUCHER'S DISEASE. Biochem Biophys Res Commun. 1965 Jan 18;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choudary P. V., Barranger J. A., Tsuji S., Mayor J., LaMarca M. E., Cepko C. L., Mulligan R. C., Ginns E. I. Retrovirus-mediated transfer of the human glucocerebrosidase gene to Gaucher fibroblasts. Mol Biol Med. 1986 Jun;3(3):293–299. [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Dinur T., Osiecki K. M., Legler G., Gatt S., Desnick R. J., Grabowski G. A. Human acid beta-glucosidase: isolation and amino acid sequence of a peptide containing the catalytic site. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1660–1664. doi: 10.1073/pnas.83.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Harris N., Brill E., Shohat O., Prokocimer M., Wolf D., Arai N., Rotter V. Molecular basis for heterogeneity of the human p53 protein. Mol Cell Biol. 1986 Dec;6(12):4650–4656. doi: 10.1128/mcb.6.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Weissmann G. Genetic disorders of lysosomes. Prog Med Genet. 1976;1:49–101. [PubMed] [Google Scholar]
- Horowitz M., Cepko C. L., Sharp P. A. Expression of chimeric genes in the early region of SV40. J Mol Appl Genet. 1983;2(2):147–159. [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matlashewski G. J., Tuck S., Pim D., Lamb P., Schneider J., Crawford L. V. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol. 1987 Feb;7(2):961–963. doi: 10.1128/mcb.7.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reiner O., Wigderson M., Horowitz M. Structural analysis of the human glucocerebrosidase genes. DNA. 1988 Mar;7(2):107–116. doi: 10.1089/dna.1988.7.107. [DOI] [PubMed] [Google Scholar]
- Reiner O., Wilder S., Givol D., Horowitz M. Efficient in vitro and in vivo expression of human glucocerebrosidase cDNA. DNA. 1987 Apr;6(2):101–108. doi: 10.1089/dna.1987.6.101. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J. A., West C., Kuhl W., Treger L., Beutler E. The human glucocerebrosidase gene has two functional ATG initiator codons. Am J Hum Genet. 1987 Dec;41(6):1016–1024. [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Kuhl W., West C., Beutler E. Gaucher disease: retrovirus-mediated correction of the enzymatic defect in cultured cells. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1041–1046. doi: 10.1101/sqb.1986.051.01.120. [DOI] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Stubblefield B. K., Mayor J. A., Barranger J. A., Ginns E. I. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N Engl J Med. 1987 Mar 5;316(10):570–575. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Winfield S., Barranger J. A., Ginns E. I. Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J Biol Chem. 1986 Jan 5;261(1):50–53. [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Barranger J. A., Stubblefield B. K., LaMarca M. E., Ginns E. I. Genetic heterogeneity in type 1 Gaucher disease: multiple genotypes in Ashkenazic and non-Ashkenazic individuals. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2349–2352. doi: 10.1073/pnas.85.7.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]