Abstract
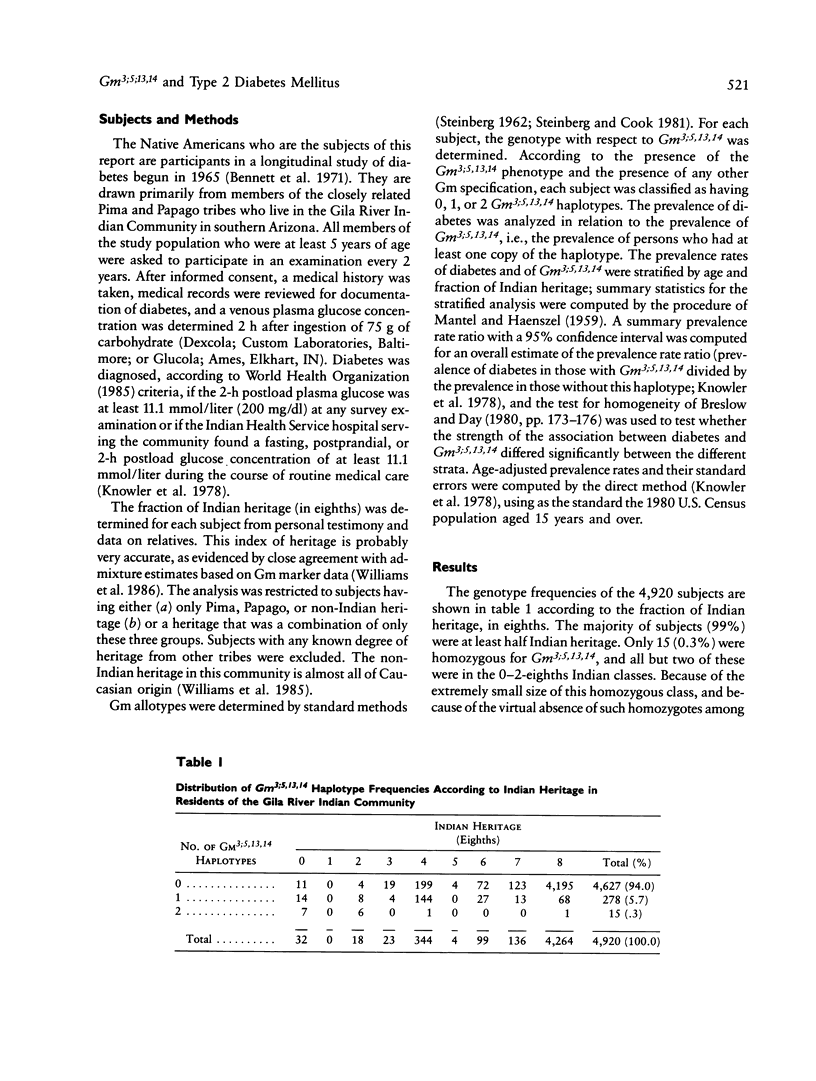
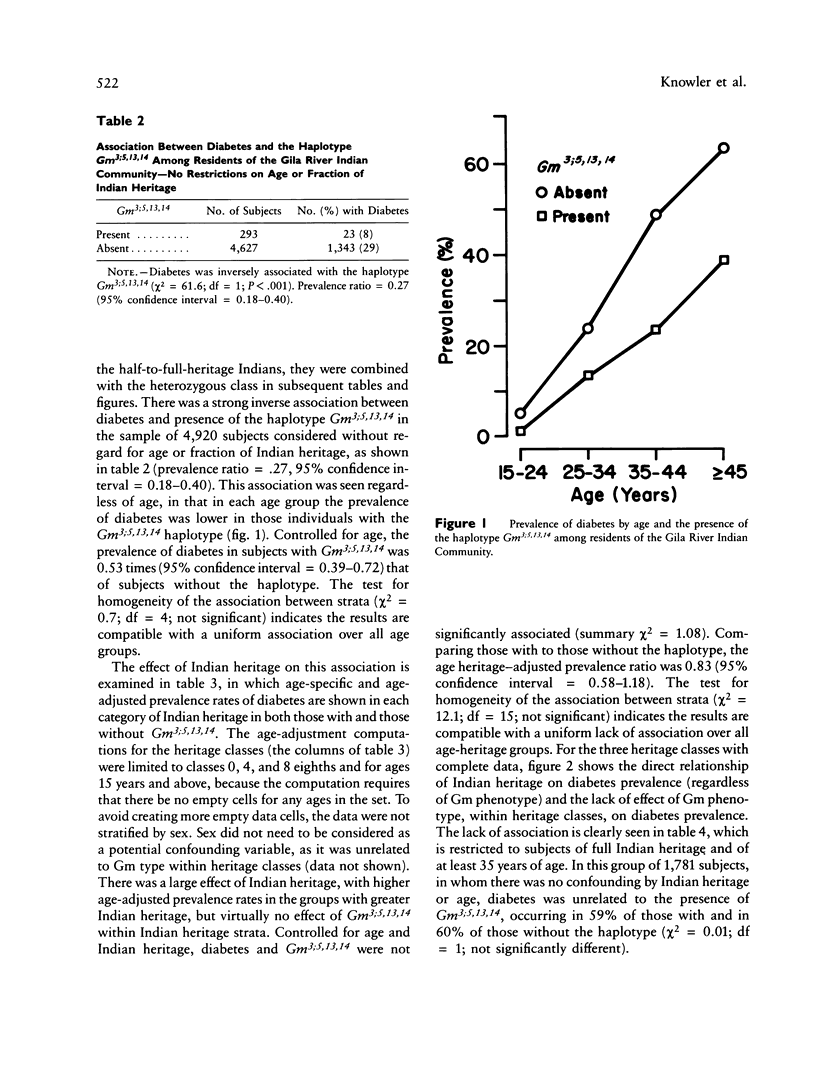
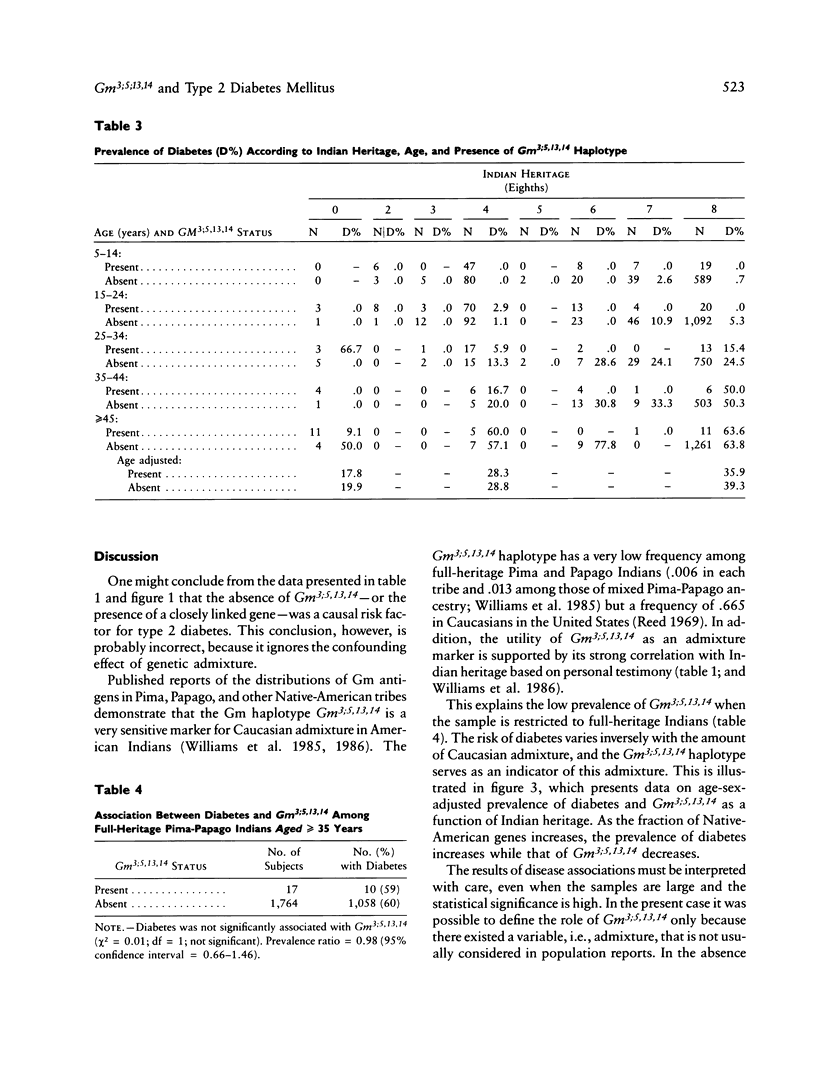
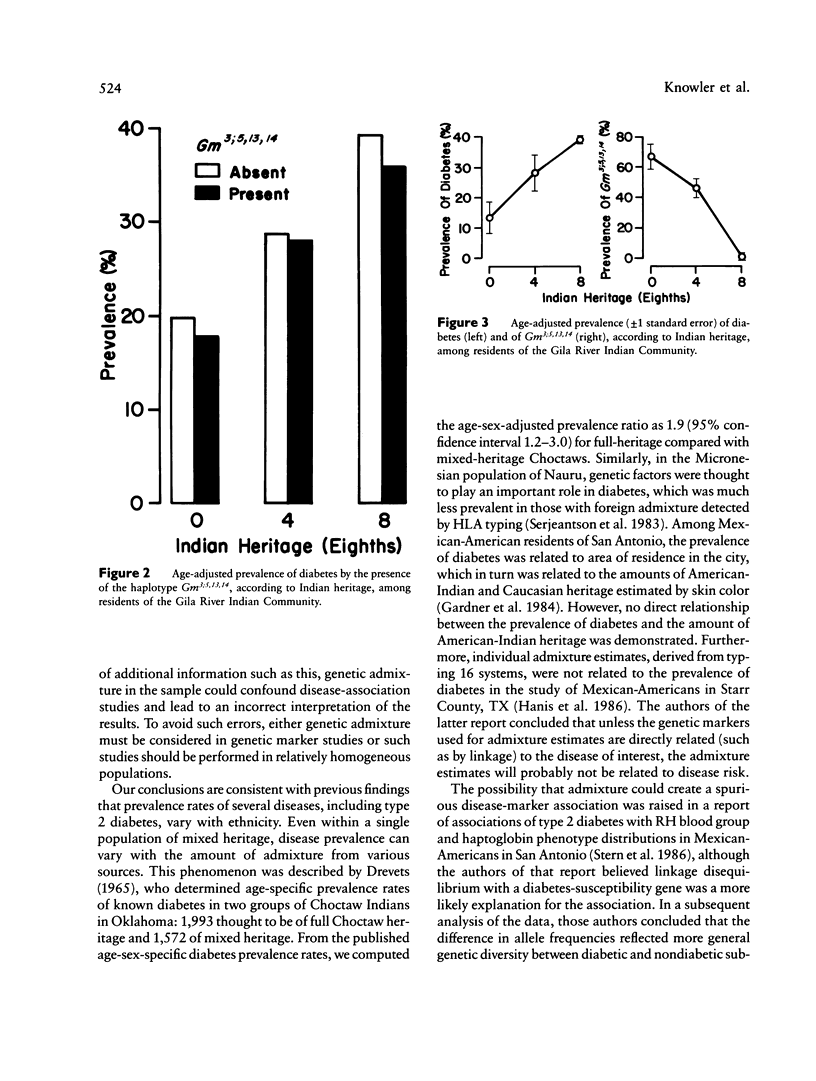
In a sample of 4,920 Native Americans of the Pima and Papago tribes, there is a very strong negative association between the Gm haplotype Gm3;5,13,14 and type 2--or non-insulin-dependent--diabetes mellitus (prevalence ratio = 0.27, 95% confidence interval 0.18-0.40). One might conclude from this observation that the absence of this haplotype--or the presence of a closely linked gene--is a causal risk factor for the disease. It is shown that Gm3;5,13,14 is a marker for Caucasian admixture, and it is most likely the presence of Caucasian alleles and the concomitant decrease of Indian alleles that lowers the risk for diabetes, rather than the direct action of the haplotype or of a closely linked locus. This study demonstrates both the potential confounding effect of admixture on the interpretation of disease association studies and the importance of considering genetic admixture (or excluding individuals with genetic admixture) in studies of genetic markers of disease. The relationship between this admixture marker and the prevalence of diabetes also suggests a strong genetic component in the susceptibility to type 2 diabetes in Pima and Papago Indians.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. D., Adams Y. J., Knight J. G., McCall J., White P., Horrocks R., van Loghem E. A solution to the genetic and environmental puzzles of insulin-dependent diabetes mellitus. Lancet. 1984 Feb 25;1(8374):420–424. doi: 10.1016/s0140-6736(84)91753-7. [DOI] [PubMed] [Google Scholar]
- Bennett P. H., Burch T. A., Miller M. Diabetes mellitus in American (Pima) Indians. Lancet. 1971 Jul 17;2(7716):125–128. doi: 10.1016/s0140-6736(71)92303-8. [DOI] [PubMed] [Google Scholar]
- Berg K., Aarseth S., Lundevall J., Reinskou T. Blood groups and genetic serum types in diabetes mellitus. Diabetologia. 1967 Mar;3(1):30–34. doi: 10.1007/BF01269908. [DOI] [PubMed] [Google Scholar]
- Briggs B. R., Jackson W. P., DuToit E. D., Botha M. C. The histocompatibility (HLA) antigen distribution in diabetes in southern African Blacks (Xhosa). Diabetes. 1980 Jan;29(1):68–71. doi: 10.2337/diab.29.1.68. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Ferrell R. E., Stern M. P., Haffner S. M., Hazuda H. P., Rosenthal M. Relationship of prevalence of non-insulin-dependent diabetes mellitus to Amerindian admixture in the Mexican Americans of San Antonio, Texas. Genet Epidemiol. 1986;3(6):435–454. doi: 10.1002/gepi.1370030608. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Weiss K. M. Frequencies of complex diseases in hybrid populations. Am J Phys Anthropol. 1986 Aug;70(4):489–503. doi: 10.1002/ajpa.1330700408. [DOI] [PubMed] [Google Scholar]
- DREVETS C. C. DIABETES MELLITUS IN CHOCTAW INDIANS. J Okla State Med Assoc. 1965 Jul;58:322–329. [PubMed] [Google Scholar]
- Field L. L., Anderson C. E., Rimoin D. L. Inheritance of immunoglobulin light-chain genes in pairs of siblings with insulin-dependent diabetes mellitus. Lancet. 1984 May 19;1(8386):1132–1132. doi: 10.1016/s0140-6736(84)92553-4. [DOI] [PubMed] [Google Scholar]
- Gardner L. I., Jr, Stern M. P., Haffner S. M., Gaskill S. P., Hazuda H. P., Relethford J. H., Eifler C. W. Prevalence of diabetes in Mexican Americans. Relationship to percent of gene pool derived from native American sources. Diabetes. 1984 Jan;33(1):86–92. doi: 10.2337/diab.33.1.86. [DOI] [PubMed] [Google Scholar]
- Hanis C. L., Chakraborty R., Ferrell R. E., Schull W. J. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Phys Anthropol. 1986 Aug;70(4):433–441. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- Knowler W. C., Bennett P. H., Hamman R. F., Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978 Dec;108(6):497–505. doi: 10.1093/oxfordjournals.aje.a112648. [DOI] [PubMed] [Google Scholar]
- Knowler W. C., Pettitt D. J., Bennett P. H., Williams R. C. Diabetes mellitus in the Pima Indians: genetic and evolutionary considerations. Am J Phys Anthropol. 1983 Sep;62(1):107–114. doi: 10.1002/ajpa.1330620114. [DOI] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Nakao Y., Matsumoto H., Miyazaki T., Mizuno N., Arima N., Wakisaka A., Okimoto K., Akazawa Y., Tsuji K., Fujita T. IgG heavy-chain (Gm) allotypes and immune response to insulin in insulin-requiring diabetes mellitus. N Engl J Med. 1981 Feb 12;304(7):407–409. doi: 10.1056/NEJM198102123040706. [DOI] [PubMed] [Google Scholar]
- Reed T. E. Critical tests of hypotheses for race mixture using Gm data on American Caucasians and Negroes. Am J Hum Genet. 1969 Jan;21(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Serjeantson S. W., Owerbach D., Zimmet P., Nerup J., Thoma K. Genetics of diabetes in Nauru: effects of foreign admixture, HLA antigens and the insulin-gene-linked polymorphism. Diabetologia. 1983 Jul;25(1):13–17. doi: 10.1007/BF00251889. [DOI] [PubMed] [Google Scholar]
- Serjeantson S. W., Ryan D. P., Ram P., Zimmet P. HLA and non-insulin dependent diabetes in Fiji indians. Med J Aust. 1981 May 2;1(9):462–464. doi: 10.5694/j.1326-5377.1981.tb135735.x. [DOI] [PubMed] [Google Scholar]
- Smouse P. E., Williams R. C. Multivariate analysis of HLA-disease associations. Biometrics. 1982 Sep;38(3):757–768. [PubMed] [Google Scholar]
- Stern M. P., Ferrell R. E., Rosenthal M., Haffner S. M., Hazuda H. P. Association between NIDDM, RH blood group, and haptoglobin phenotype. Results from the San Antonio Heart Study. Diabetes. 1986 Apr;35(4):387–391. doi: 10.2337/diab.35.4.387. [DOI] [PubMed] [Google Scholar]
- Svejgaard A., Jersild C., Nielsen L. S., Bodmer W. F. HL-A antigens and disease. Statistical and genetical considerations. Tissue Antigens. 1974;4(2):95–105. doi: 10.1111/j.1399-0039.1974.tb00230.x. [DOI] [PubMed] [Google Scholar]
- WOOLF B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955 Jun;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Knowler W. C., Butler W. J., Pettitt D. J., Lisse J. R., Bennett P. H., Mann D. L., Johnson A. H., Terasaki P. I. HLA-A2 and type 2 (insulin independent) diabetes mellitus in Pima Indians: an association of allele frequency with age. Diabetologia. 1981 Nov;21(5):460–463. doi: 10.1007/BF00257786. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Steinberg A. G., Gershowitz H., Bennett P. H., Knowler W. C., Pettitt D. J., Butler W., Baird R., Dowda-Rea L., Burch T. A. GM allotypes in Native Americans: evidence for three distinct migrations across the Bering land bridge. Am J Phys Anthropol. 1985 Jan;66(1):1–19. doi: 10.1002/ajpa.1330660102. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Steinberg A. G., Knowler W. C., Pettitt D. J. Gm 3;5,13,14 and stated-admixture: independent estimates of admixture in American Indians. Am J Hum Genet. 1986 Sep;39(3):409–413. [PMC free article] [PubMed] [Google Scholar]



