Abstract
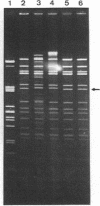
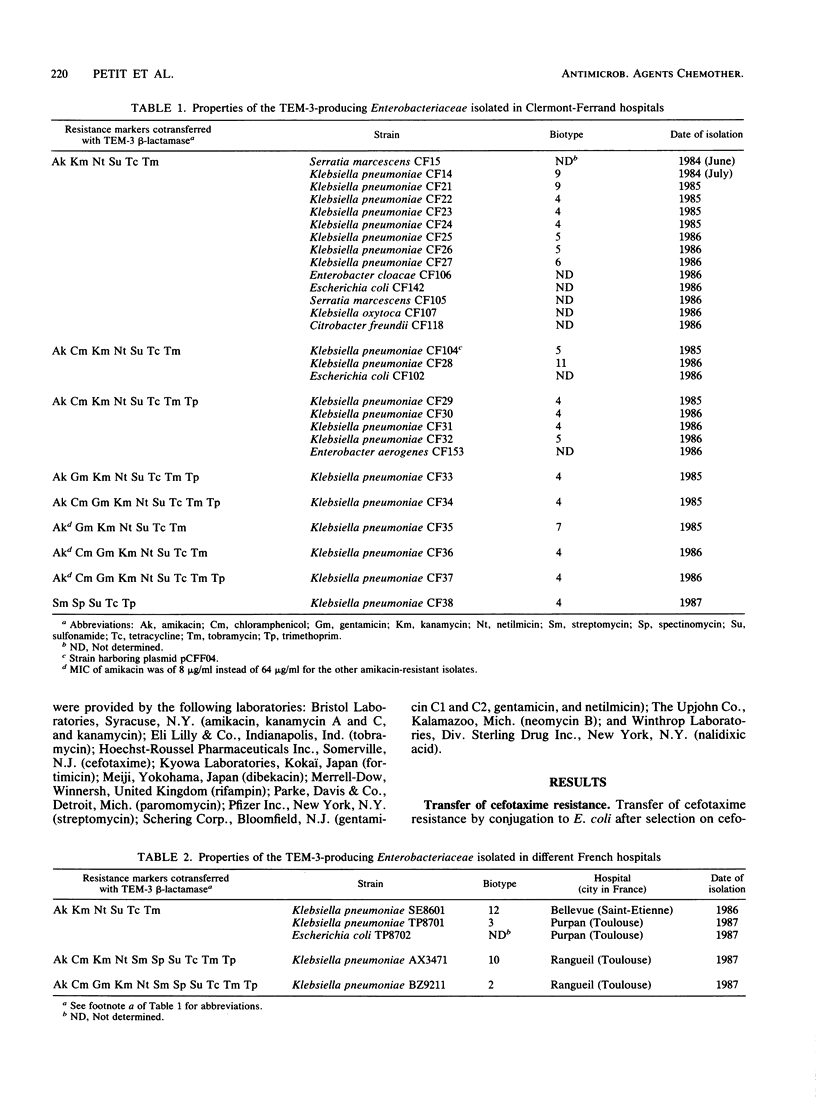
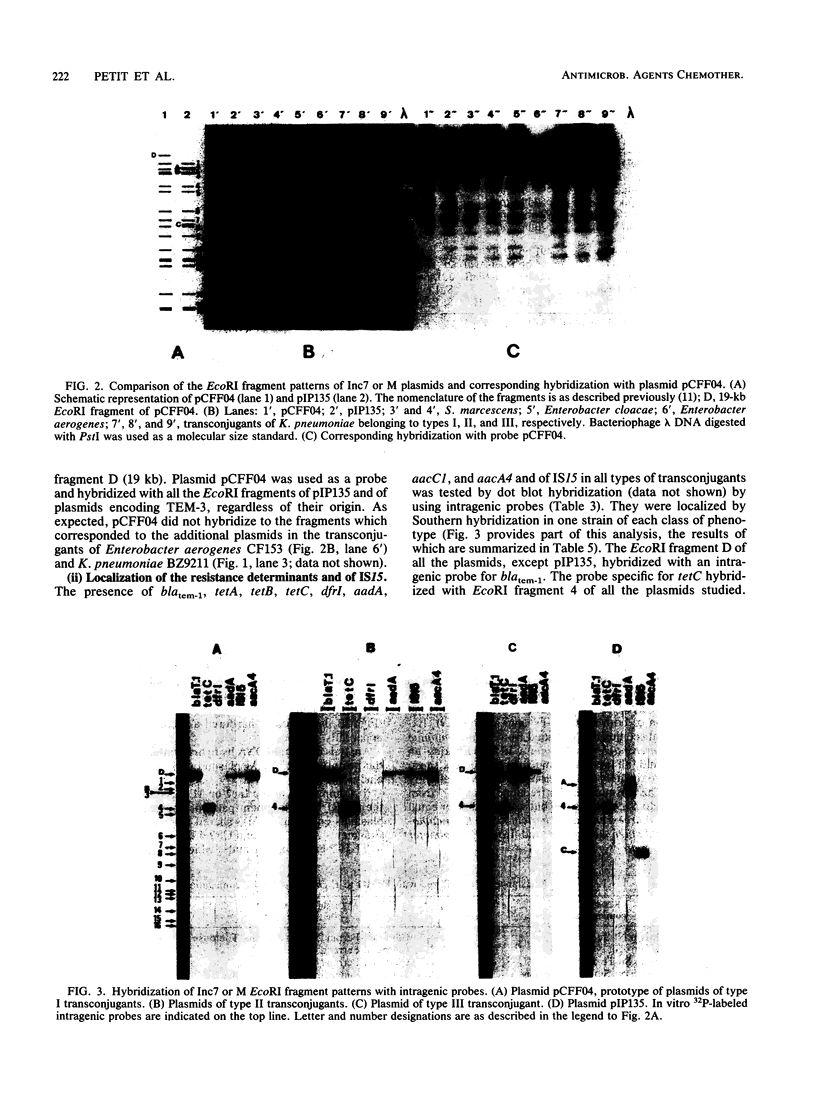
A total of 33 clinical isolates encoding TEM-3 (CTX-1) from four French hospitals were studied. The strains belonged to seven species, Klebsiella pneumoniae (n = 24), Escherichia coli (n = 3), Serratia marcescens (n = 2), Citrobacter freundii (n = 1), Enterobacter aerogenes (n = 1), Enterobacter cloacae (n = 1), and Klebsiella oxytoca (n = 1). All the strains harbored an Inc7 or M self-transferable plasmid with a size of approximately 85 kilobases. The plasmids had closely related EcoRI, HincII, HindIII, and PvuII restriction endonuclease-generated patterns and conferred resistance to all beta-lactams, except cephamycins and imipenem; to tetracycline, because of the presence of the genes blatem-3 and tetC, respectively, as determined by hybridization with specific probes; and to sulfonamide. Depending on the presence or absence and level of expression of the genes aacA4, aadA, and dfrI and of insertion element IS15, four types of plasmids could be distinguished. Plasmid pCFF04, the prototype plasmid encoding TEM-3, was widespread and appeared, by Southern hybridization, as the progenitor of the other types of replicons. The plasmid epidemic responsible for dissemination of TEM-3 in clinical isolates of members of the family Enterobacteriaceae may have originated in S. marcescens since pCFF04 was first detected in this species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C., Legrand P., Philippon A., Montravers F., Ansquer M., Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987 Aug 8;2(8554):302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Weisblum B., Davies J. Aminoglycoside-modifying enzyme of an antibiotic-producing bacterium acts as a determinant of antibiotic resistance in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):999–1003. doi: 10.1073/pnas.74.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hollingshead S., Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid. 1985 Jan;13(1):17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Kliebe C., Nies B. A., Meyer J. F., Tolxdorff-Neutzling R. M., Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe H., Shah P., Krcmery V., Antal M., Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983 Nov-Dec;11(6):315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Courvalin P. IS15, a new insertion sequence widely spread in R plasmids of gram-negative bacteria. Mol Gen Genet. 1983;189(1):102–112. doi: 10.1007/BF00326061. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Witchitz J., Courvalin P. Modular evolution of disseminated Inc 7-M plasmids encoding gentamicin resistance. Plasmid. 1982 Nov;8(3):215–231. doi: 10.1016/0147-619x(82)90060-9. [DOI] [PubMed] [Google Scholar]
- Lesage D. D., Gerbaud G. R., Chabbert Y. A. Carte génétique et strucutre chez Escherichia coli K12 d'un plasmide de résistance isolé de Salmonella ordonez. Ann Microbiol (Paris) 1975 May-Jun;126A(4):435–448. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K. H., Hopkins J. D., Gilleece E. S., Chao L., O'Brien T. F. Molecular evolution, species distribution, and clinical consequences of an endemic aminoglycoside resistance plasmid. Antimicrob Agents Chemother. 1986 Apr;29(4):628–633. doi: 10.1128/aac.29.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Meyer J. F., Wiedemann B. Characterization of aminoglycoside 6'-N-acetyltransferases [AAC(6')] from gram-negative bacteria and Streptomyces kanamyceticus. J Antimicrob Chemother. 1985 Mar;15(3):271–282. doi: 10.1093/jac/15.3.271. [DOI] [PubMed] [Google Scholar]
- Nobuta K., Tolmasky M. E., Crosa L. M., Crosa J. H. Sequencing and expression of the 6'-N-acetyltransferase gene of transposon Tn1331 from Klebsiella pneumoniae. J Bacteriol. 1988 Aug;170(8):3769–3773. doi: 10.1128/jb.170.8.3769-3773.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. F., Ross D. G., Guzman M. A., Medeiros A. A., Hedges R. W., Botstein D. Dissemination of an antibiotic resistance plasmid in hospital patient flora. Antimicrob Agents Chemother. 1980 Apr;17(4):537–543. doi: 10.1128/aac.17.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Sirot J., Chanal C., Petit A., Sirot D., Labia R., Gerbaud G. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated beta-lactamases markedly active against third-generation cephalosporins: epidemiologic studies. Rev Infect Dis. 1988 Jul-Aug;10(4):850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]
- Sougakoff W., Goussard S., Gerbaud G., Courvalin P. Plasmid-mediated resistance to third-generation cephalosporins caused by point mutations in TEM-type penicillinase genes. Rev Infect Dis. 1988 Jul-Aug;10(4):879–884. doi: 10.1093/clinids/10.4.879. [DOI] [PubMed] [Google Scholar]
- Tenover F. C., Filpula D., Phillips K. L., Plorde J. J. Cloning and sequencing of a gene encoding an aminoglycoside 6'-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988 Jan;170(1):471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran van Nhieu G., Collatz E. Primary structure of an aminoglycoside 6'-N-acetyltransferase AAC(6')-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987 Dec;169(12):5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nhieu G. T., Goldstein F. W., Pinto M. E., Acar J. F., Collatz E. Transfer of amikacin resistance by closely related plasmids in members of the family Enterobacteriaceae isolated in Chile. Antimicrob Agents Chemother. 1986 May;29(5):833–837. doi: 10.1128/aac.29.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]