Abstract
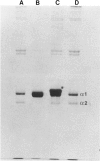
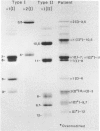
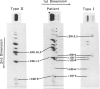
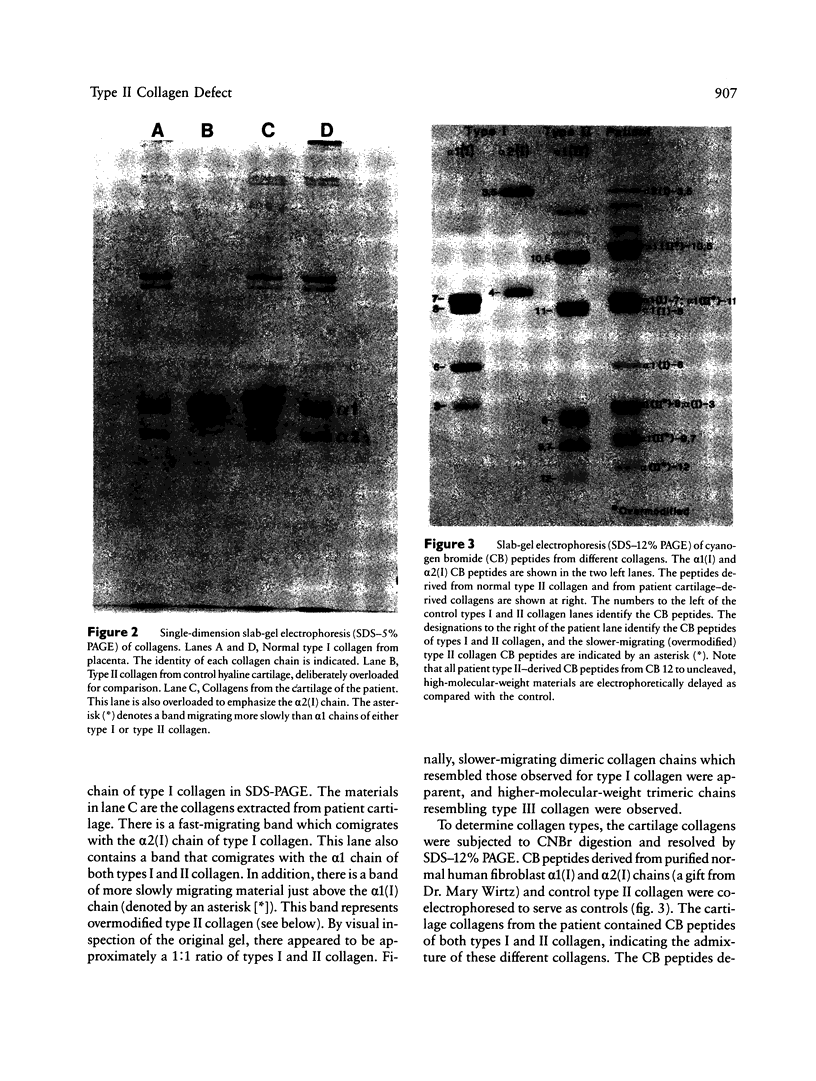
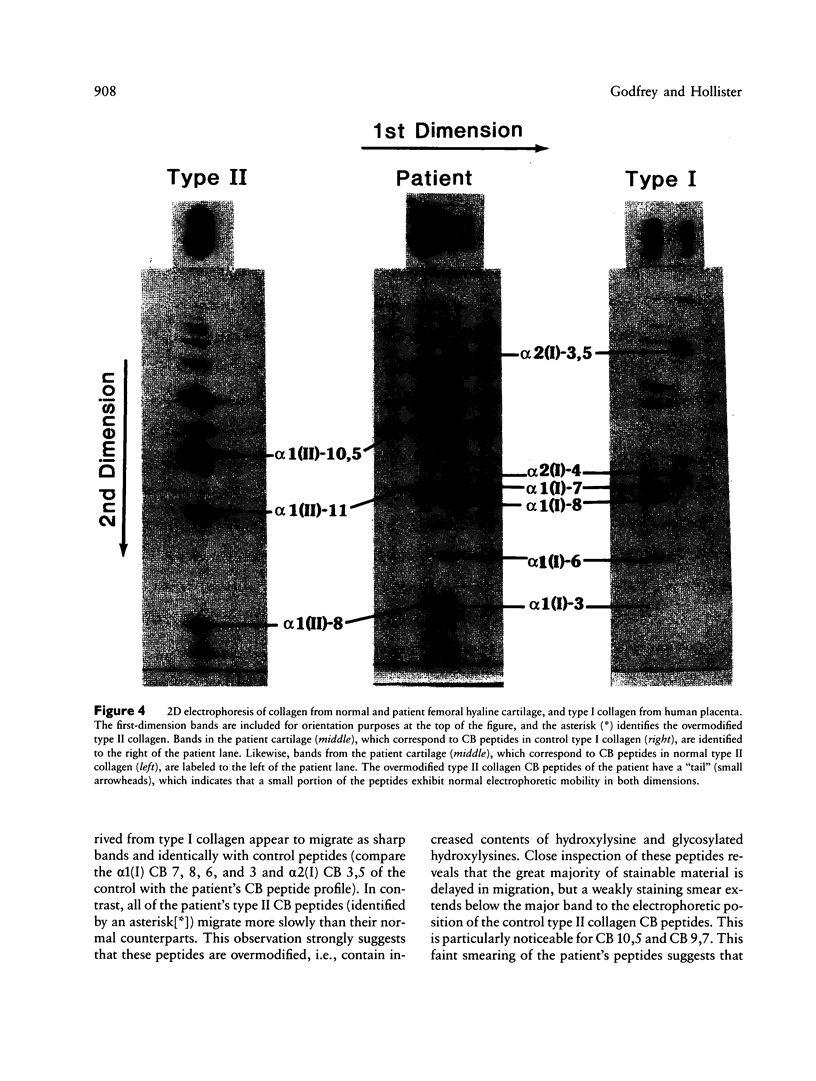
We have extended the study of a mild case of type II achondrogenesis-hypochondrogenesis to include biochemical analyses of cartilage, bone, and the collagens produced by dermal fibroblasts. Type I collagen extracted from bone and types I and III collagen produced by dermal fibroblasts were normal, as was the hexosamine ratio of cartilage proteoglycans. Hyaline cartilage, however, contained approximately equal amounts of types I and II collagen and decreased amounts of type XI collagen. Unlike the normal SDS-PAGE mobility. Two-dimensional SDS-PAGE revealed extensive overmodification of all type II cyanogen bromide peptides in a pattern consistent with heterozygosity for an abnormal pro alpha 1(II) chain which impaired the assembly and/or folding of type II collagen. This interpretation implies that dominant mutations of the COL2A1 gene may cause type II achondrogenesis-hypochondrogenesis. More generally, emerging data implicating defects of type II collagen in the type II achondrogenesis-hypochondrogenesis-spondyloepiphyseal dysplasia congenita spectrum and in the Kniest-Stickler syndrome spectrum suggest that diverse mutations of this gene may be associated with widely differing phenotypic outcome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Peterson K. E., Byers P. H. Peptide mapping of collagen chains using CNBr cleavage of proteins within polyacrylamide gels. Coll Relat Res. 1981 Nov;1(6):543–548. doi: 10.1016/s0174-173x(81)80035-0. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Borochowitz Z., Ornoy A., Lachman R., Rimoin D. L. Achondrogenesis II-hypochondrogenesis: variability versus heterogeneity. Am J Med Genet. 1986 Jun;24(2):273–288. doi: 10.1002/ajmg.1320240208. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Holbrook K. A., McGillivray B., MacLeod P. M., Lowry R. B. Clinical and ultrastructural heterogeneity of type IV Ehlers-Danlos syndrome. Hum Genet. 1979 Mar 12;47(2):141–150. doi: 10.1007/BF00273196. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Bächinger H. P., Fessler L. I., Timpl R., Fessler J. H. Chain assembly intermediate in the biosynthesis of type III procollagen in chick embryo blood vessels. J Biol Chem. 1981 Dec 25;256(24):13193–13199. [PubMed] [Google Scholar]
- Clark J. G., Kuhn C., 3rd, Uitto J. Lung collagen in type IV Ehlers-Danlos syndrome: ultrastructural and biochemical studies. Am Rev Respir Dis. 1980 Dec;122(6):971–978. doi: 10.1164/arrd.1980.122.6.971. [DOI] [PubMed] [Google Scholar]
- Click E. M., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha 1 and alpha 2 chains of human skin collagen. Biochemistry. 1970 Nov 24;9(24):4699–4706. doi: 10.1021/bi00826a012. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Scott R. D., Miller E. J., Piez K. A. Isolation and characterization of the peptides derived from soluble human and baboon skin collagen after cyanogen bromide cleavage. J Biol Chem. 1971 Mar 25;246(6):1718–1724. [PubMed] [Google Scholar]
- Eyre D. R., Upton M. P., Shapiro F. D., Wilkinson R. H., Vawter G. F. Nonexpression of cartilage type II collagen in a case of Langer-Saldino achondrogenesis. Am J Hum Genet. 1986 Jul;39(1):52–67. [PMC free article] [PubMed] [Google Scholar]
- Godfrey M., Keene D. R., Blank E., Hori H., Sakai L. Y., Sherwin L. A., Hollister D. W. Type II achondrogenesis-hypochondrogenesis: morphologic and immunohistopathologic studies. Am J Hum Genet. 1988 Dec;43(6):894–903. [PMC free article] [PubMed] [Google Scholar]
- Horton W. A., Chou J. W., Machado M. A. Cartilage collagen analysis in the chondrodystrophies. Coll Relat Res. 1985 Sep;5(4):349–354. doi: 10.1016/s0174-173x(85)80023-6. [DOI] [PubMed] [Google Scholar]
- Horton W. A. Histochemistry, a valuable tool in connective tissue research. Coll Relat Res. 1984 May;4(3):231–237. doi: 10.1016/s0174-173x(84)80045-x. [DOI] [PubMed] [Google Scholar]
- Horton W. A., Machado M. A., Chou J. W., Campbell D. Achondrogenesis type II, abnormalities of extracellular matrix. Pediatr Res. 1987 Sep;22(3):324–329. doi: 10.1203/00006450-198709000-00017. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maroteaux P., Stanescu V., Stanescu R. The lethal chondrodysplasias. Clin Orthop Relat Res. 1976 Jan-Feb;(114):31–45. [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Morris N. P., Bächinger H. P. Type XI collagen is a heterotrimer with the composition (1 alpha, 2 alpha, 3 alpha) retaining non-triple-helical domains. J Biol Chem. 1987 Aug 15;262(23):11345–11350. [PubMed] [Google Scholar]
- Morris N. P., Keene D. R., Glanville R. W., Bentz H., Burgeson R. E. The tissue form of type VII collagen is an antiparallel dimer. J Biol Chem. 1986 Apr 25;261(12):5638–5644. [PubMed] [Google Scholar]
- Murray L. W., Rimoin D. L. Abnormal type II collagen in the spondyloepiphyseal dysplasias. Pathol Immunopathol Res. 1988;7(1-2):99–103. doi: 10.1159/000157111. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L., Hollister D., Murray L., Rimoin D. Kniest dysplasia is characterized by an apparent abnormal processing of the C-propeptide of type II cartilage collagen resulting in imperfect fibril assembly. J Clin Invest. 1988 Feb;81(2):579–589. doi: 10.1172/JCI113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J. Osteogenesis imperfecta: phenotypic heterogeneity, protein suicide, short and long collagen. Am J Hum Genet. 1984 May;36(3):499–505. [PMC free article] [PubMed] [Google Scholar]
- Spranger J. Pattern recognition in bone dysplasias. Prog Clin Biol Res. 1985;200:315–342. [PubMed] [Google Scholar]
- Steinmann B., Nicholls A., Pope F. M. Clinical variability of osteogenesis imperfecta reflecting molecular heterogeneity: cysteine substitutions in the alpha 1(I) collagen chain producing lethal and mild forms. J Biol Chem. 1986 Jul 5;261(19):8958–8964. [PubMed] [Google Scholar]
- Steinmann B., Rao V. H., Vogel A., Bruckner P., Gitzelmann R., Byers P. H. Cysteine in the triple-helical domain of one allelic product of the alpha 1(I) gene of type I collagen produces a lethal form of osteogenesis imperfecta. J Biol Chem. 1984 Sep 10;259(17):11129–11138. [PubMed] [Google Scholar]
- Stolle C. A., Pyeritz R. E., Myers J. C., Prockop D. J. Synthesis of an altered type III procollagen in a patient with type IV Ehlers-Danlos syndrome. A structural change in the alpha 1(III) chain which makes the protein more susceptible to proteinases. J Biol Chem. 1985 Feb 10;260(3):1937–1944. [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- Whitley C. B., Gorlin R. J. Achondrogenesis: new nosology with evidence of genetic heterogeneity. Radiology. 1983 Sep;148(3):693–698. doi: 10.1148/radiology.148.3.6878687. [DOI] [PubMed] [Google Scholar]
- Williams C. J., Prockop D. J. Synthesis and processing of a type I procollagen containing shortened pro-alpha 1(I) chains by fibroblasts from a patient with osteogenesis imperfecta. J Biol Chem. 1983 May 10;258(9):5915–5921. [PubMed] [Google Scholar]