Abstract
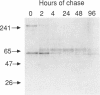
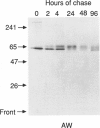
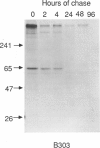
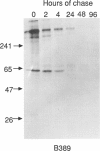
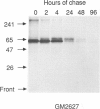
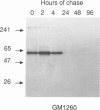
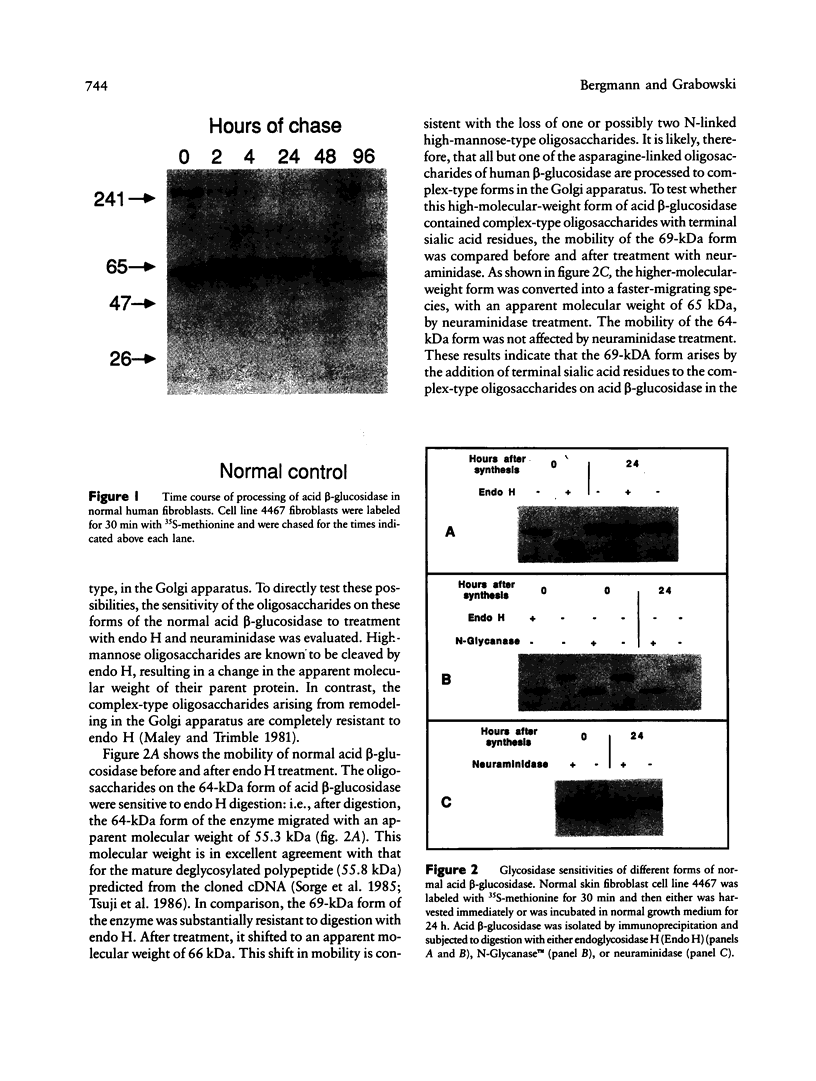
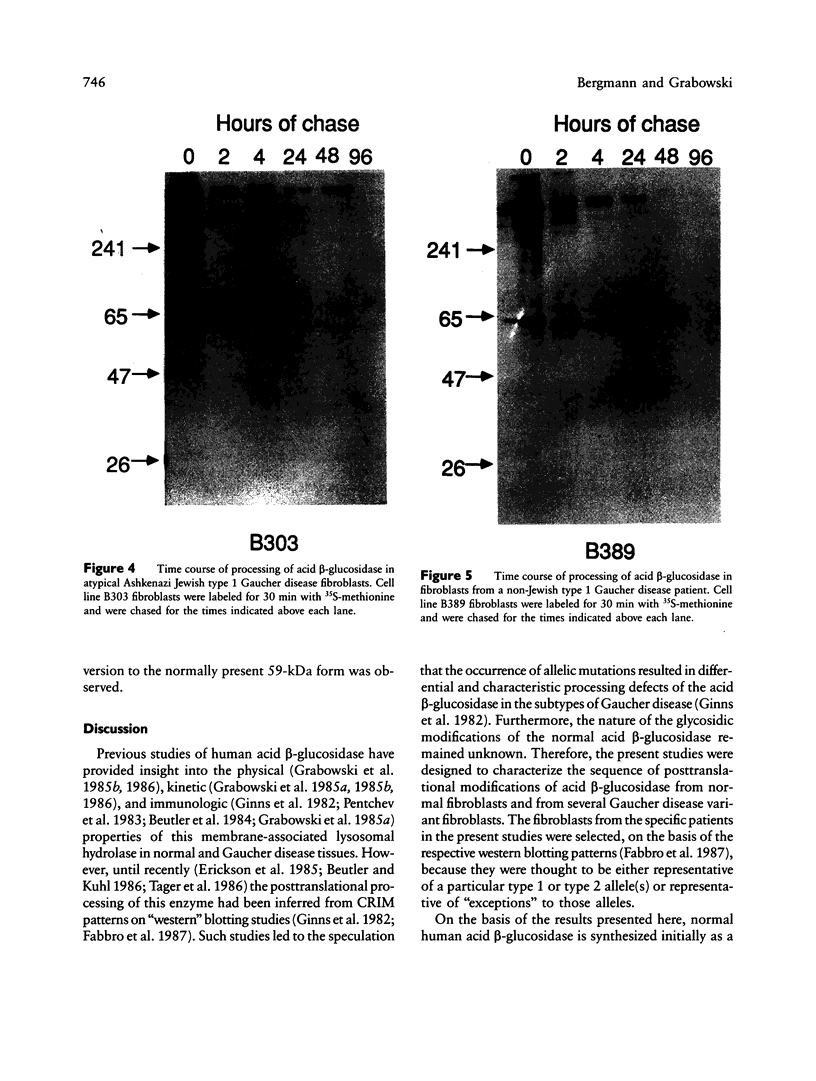
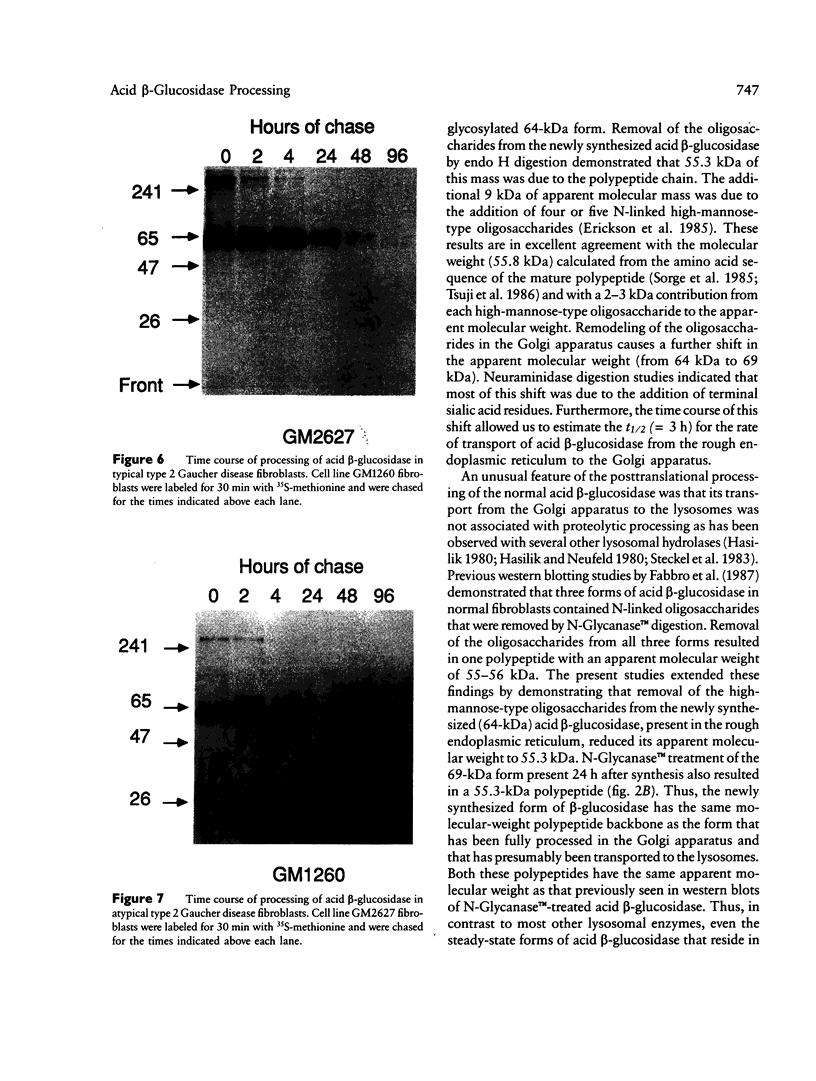
The major processing steps in the maturation of the lysosomal hydrolase, acid beta-glucosidase, were examined in fibroblasts from normal individuals and from patients with types 1 and 2 Gaucher disease. In pulse-chase studies with normal fibroblasts, remodeling of N-linked oligosaccharides resulted in the temporal appearance of three molecular-weight forms of acid beta-glucosidase. An initial 64-kDa form, containing high mannose-type oligosaccharide side chains, was processed quantitatively, within 24 h, to a sialylated 69-kDa form. During the subsequent 96 h, some of the 69-kDa form is processed to 59 kDa. Glycosidase digestion studies revealed that the increase in the apparent molecular weight of the normal enzyme from 64 kDa to 69 kDa resulted primarily from the addition to sialic acid residues in the Golgi apparatus. The polypeptide backbone of both the 64-kDa and 69-kDa forms was 55.3 kDa. Processing of acid beta-glucosidase in fibroblasts from three of four type 1 (nonneuronopathic) Ashkenazi Jewish Gaucher disease patients was nearly normal. With fibroblasts from one Ashkenazi Jewish and three non-Jewish type 1 as well as from two type 2 (acute neuronopathic) Gaucher disease patients, only a 64-kDa form of acid beta-glucosidase was detected. Inefficient and incomplete processing to the 69-kDa form was found in one type 2 cell line (GM2627). These results indicate that no firm correlation exists between the type or degree of abnormal processing of acid beta-glucosidase in fibroblasts and the phenotype of Gaucher disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY R. O., KANFER J. N., SHAPIRO D. METABOLISM OF GLUCOCEREBROSIDES. II. EVIDENCE OF AN ENZYMATIC DEFICIENCY IN GAUCHER'S DISEASE. Biochem Biophys Res Commun. 1965 Jan 18;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W. Glucocerebrosidase processing in normal fibroblasts and in fibroblasts from patients with type I, type II, and type III Gaucher disease. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7472–7474. doi: 10.1073/pnas.83.19.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Kuhl W., Sorge J. Cross-reacting material in Gaucher disease fibroblasts. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6506–6510. doi: 10.1073/pnas.81.20.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick R. J. Gaucher disease: a century of delineation and understanding. Prog Clin Biol Res. 1982;95:1–30. [PubMed] [Google Scholar]
- Erickson A. H., Ginns E. I., Barranger J. A. Biosynthesis of the lysosomal enzyme glucocerebrosidase. J Biol Chem. 1985 Nov 15;260(26):14319–14324. [PubMed] [Google Scholar]
- Fabbro D., Desnick R. J., Grabowski G. A. Gaucher disease: genetic heterogeneity within and among the subtypes detected by immunoblotting. Am J Hum Genet. 1987 Jan;40(1):15–31. [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginns E. I., Brady R. O., Pirruccello S., Moore C., Sorrell S., Furbish F. S., Murray G. J., Tager J., Barranger J. A. Mutations of glucocerebrosidase: discrimination of neurologic and non-neurologic phenotypes of Gaucher disease. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5607–5610. doi: 10.1073/pnas.79.18.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Dinur T., Osiecki K. M., Kruse J. R., Legler G., Gatt S. Gaucher disease types 1, 2, and 3: differential mutations of the acid beta-glucosidase active site identified with conduritol B epoxide derivatives and sphingosine. Am J Hum Genet. 1985 May;37(3):499–510. [PMC free article] [PubMed] [Google Scholar]
- Grabowski G. A., Goldblatt J., Dinur T., Kruse J., Svennerholm L., Gatt S., Desnick R. J. Genetic heterogeneity in Gaucher disease: physicokinetic and immunologic studies of the residual enzyme in cultured fibroblasts from non-neuronopathic and neuronopathic patients. Am J Med Genet. 1985 Jul;21(3):529–549. doi: 10.1002/ajmg.1320210316. [DOI] [PubMed] [Google Scholar]
- Grabowski G. A., Osiecki-Newman K., Dinur T., Fabbro D., Legler G., Gatt S., Desnick R. J. Human acid beta-glucosidase. Use of conduritol B epoxide derivatives to investigate the catalytically active normal and Gaucher disease enzymes. J Biol Chem. 1986 Jun 25;261(18):8263–8269. [PubMed] [Google Scholar]
- Gravel R. A., Leung A. Complementation analysis in Gaucher disease using single cell microassay techniques. Evidence for a single "Gaucher gene". Hum Genet. 1983;65(2):112–116. doi: 10.1007/BF00286645. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Grabowski G. A., Eisner R., Palese P., Smith F. I. Gaucher disease type 1: cloning and characterization of a cDNA encoding acid beta-glucosidase from an Ashkenazi Jewish patient. DNA. 1988 Oct;7(8):521–528. doi: 10.1089/dna.1.1988.7.521. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Grabowski G. A., Ludman M. D., Palese P., Smith F. I. Human acid beta-glucosidase: Northern blot and S1 nuclease analysis of mRNA from HeLa cells and normal and Gaucher disease fibroblasts. Am J Hum Genet. 1986 Dec;39(6):763–774. [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maley F., Trimble R. B. Revision of the structure for an endo-beta-N-acetylglucosaminidase H substrate using a novel modification of the Smith degradation. J Biol Chem. 1981 Feb 10;256(3):1088–1090. [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Mueller O. T., Rosenberg A. beta-Glucoside hydrolase activity of normal and glucosylceramidotic cultured human skin fibroblasts. J Biol Chem. 1977 Feb 10;252(3):825–829. [PubMed] [Google Scholar]
- Obijeski J. F., Marchenko A. T., Bishop D. H., Cann B. W., Murphy F. A. Comparative electrophoretic analysis of the virus proteins of four rhabdoviruses. J Gen Virol. 1974 Jan;22(1):21–33. doi: 10.1099/0022-1317-22-1-21. [DOI] [PubMed] [Google Scholar]
- Parkin J. L., Brunning R. D. Pathology of the Gaucher cell. Prog Clin Biol Res. 1982;95:151–175. [PubMed] [Google Scholar]
- Pentchev P. G., Neumeyer B., Svennerholm L., Groth C. G., Brady R. O. Immunological and catalytic quantitation of splenic glucocerebrosidase from the three clinical forms of Gaucher disease. Am J Hum Genet. 1983 Jul;35(4):621–628. [PMC free article] [PubMed] [Google Scholar]
- Rogalski A. A., Bergmann J. E., Singer S. J. Effect of microtubule assembly status on the intracellular processing and surface expression of an integral protein of the plasma membrane. J Cell Biol. 1984 Sep;99(3):1101–1109. doi: 10.1083/jcb.99.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Meier E. Primary structure of the vesicular stomatitis virus polymerase (L) gene: evidence for a high frequency of mutations. J Virol. 1984 Aug;51(2):505–514. doi: 10.1128/jvi.51.2.505-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., West C., Westwood B., Beutler E. Molecular cloning and nucleotide sequence of human glucocerebrosidase cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7289–7293. doi: 10.1073/pnas.82.21.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckel F., Hasilik A., von Figura K. Biosynthesis and maturation of arylsulfatase B in normal and mutant cultured human fibroblasts. J Biol Chem. 1983 Dec 10;258(23):14322–14326. [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Stubblefield B. K., Mayor J. A., Barranger J. A., Ginns E. I. A mutation in the human glucocerebrosidase gene in neuronopathic Gaucher's disease. N Engl J Med. 1987 Mar 5;316(10):570–575. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Winfield S., Barranger J. A., Ginns E. I. Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J Biol Chem. 1986 Jan 5;261(1):50–53. [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Barranger J. A., Stubblefield B. K., LaMarca M. E., Ginns E. I. Genetic heterogeneity in type 1 Gaucher disease: multiple genotypes in Ashkenazic and non-Ashkenazic individuals. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2349–2352. doi: 10.1073/pnas.85.7.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D. A., Roth S., Kudoh T., Grover W. D., Tucker S. H., Kaye E. M., Ullman M. D. Biochemical studies in a patient with subacute neuropathic Gaucher disease without visceral glucosylceramide storage. Pediatr Res. 1983 May;17(5):344–348. doi: 10.1203/00006450-198305000-00007. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Comparison of structural polypeptides from vesicular stomatitis virus (Indiana and New Jersey serotypes) and Cocal virus. J Gen Virol. 1972 Jul;16(1):1–10. doi: 10.1099/0022-1317-16-1-1. [DOI] [PubMed] [Google Scholar]