Abstract
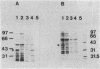
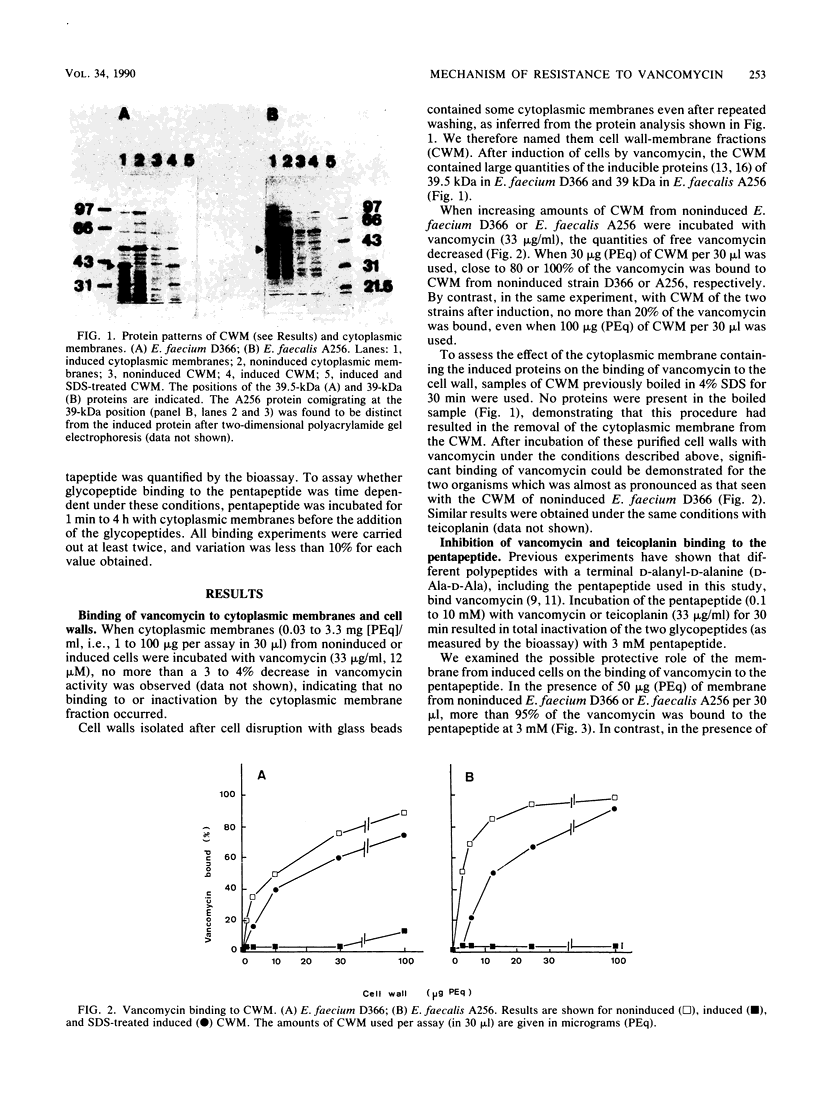
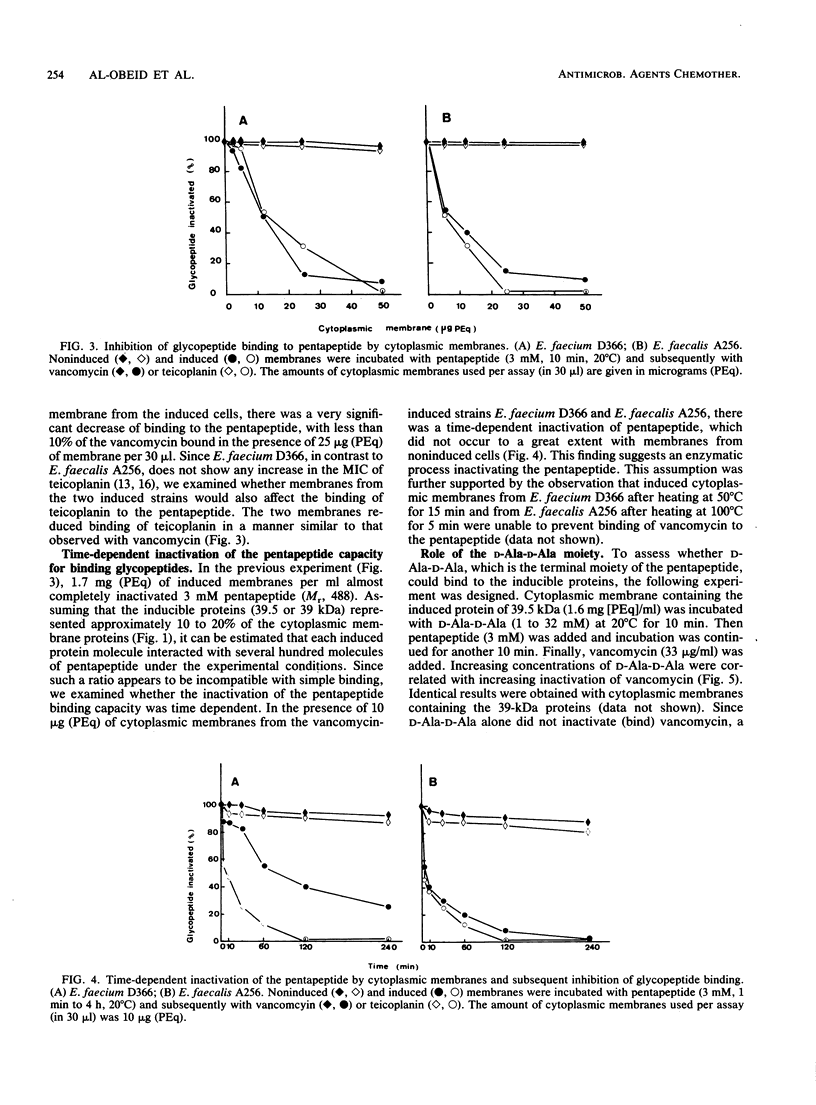
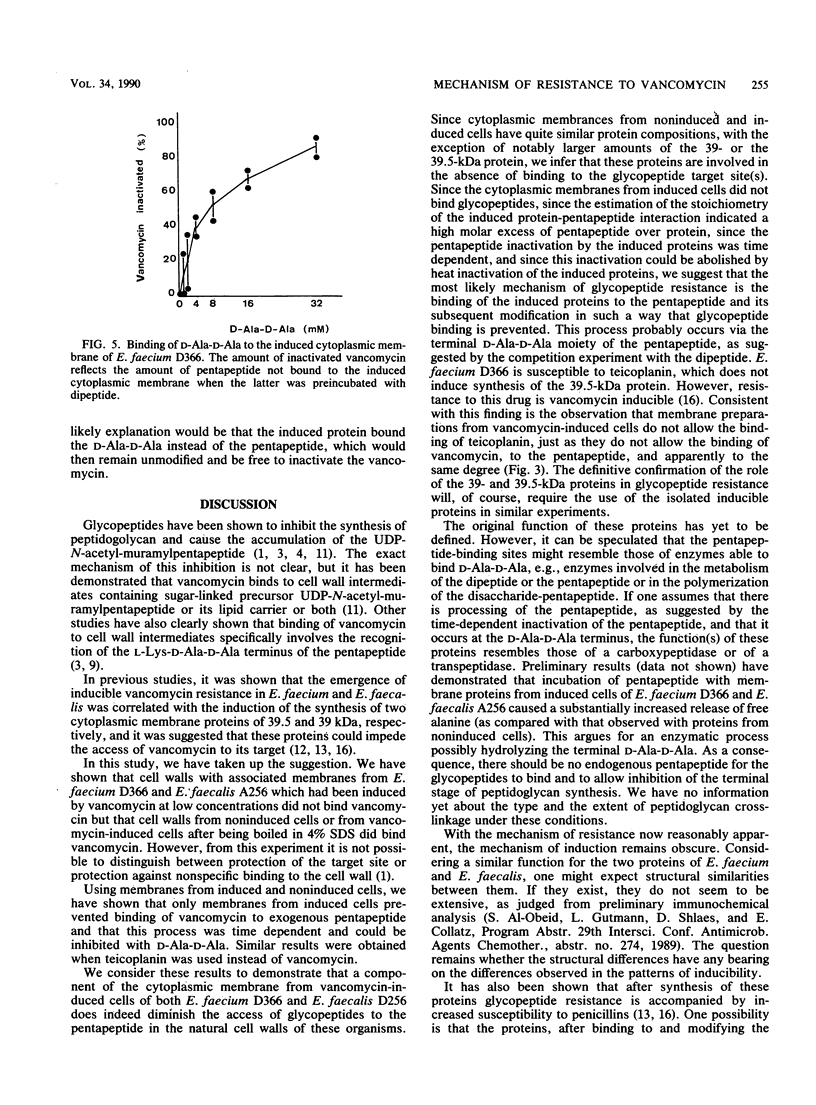
The role of the glycopeptide-inducible proteins of Enterococcus faecium D366 (39.5 kilodaltons) and Enterococcus faecalis A256 (39 kilodaltons) in the mechanism of resistance to vancomycin and teicoplanin was examined. Crude cell walls from noninduced cells or from induced cells treated with sodium dodecyl sulfate to remove the inducible proteins were shown to bind vancomycin, in contrast to cell walls containing the cytoplasmic membrane-associated induced proteins, which did not bind vancomycin. Cytoplasmic membranes from vancomycin-induced cells did not inactivate (bind) vancomycin or teicoplanin, but they could protect the glycopeptides from being bound to the synthetic pentapeptide. This protection could be competitively abolished by D-alanyl-D-alanine. A decrease in glycopeptide binding to the pentapeptide was observed in a time-dependent fashion after treatment of the pentapeptide with the cytoplasmic membranes from induced cells. We hypothesize that the inducible proteins are responsible for glycopeptide resistance due to the binding to, and subsequent enzymatic modification of, the pentapeptide precursor of peptidoglycan, which is considered to be the natural target of glycopeptides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barna J. C., Williams D. H. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. N., Perkins H. R. Compounds formed between nucleotides related to the biosynthesis of bacterial cell wall and vancomycin. Biochem Biophys Res Commun. 1966 Aug 12;24(3):489–494. doi: 10.1016/0006-291x(66)90188-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Wu C. Y., Hobbs J. N., Jr, Preston D. A., Allen N. E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Jul;33(7):1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Reynolds P. E. Reversal by a specific peptide (diacetyl-alpha gamma-L-diaminobutyryl-D-alanyl-D-alanine) of vancomycin inhibition in intact bacteria and cell-free preparations. Biochem J. 1972 Jan;126(1):139–149. doi: 10.1042/bj1260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J. 1969 Jan;111(2):195–205. doi: 10.1042/bj1110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Al-Obeid S., Shlaes J. H., Williamson R. Activity of various glycopeptides against an inducibly vancomycin-resistant strain of Enterococcus faecium (D366). J Infect Dis. 1989 Jun;159(6):1132–1135. doi: 10.1093/infdis/159.6.1132. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somma S., Gastaldo L., Corti A. Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob Agents Chemother. 1984 Dec;26(6):917–923. doi: 10.1128/aac.26.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., Collins C. H., Naidoo J., George R. C. Vancomycin-resistant enterococci. Lancet. 1988 Jan 2;1(8575-6):57–58. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]