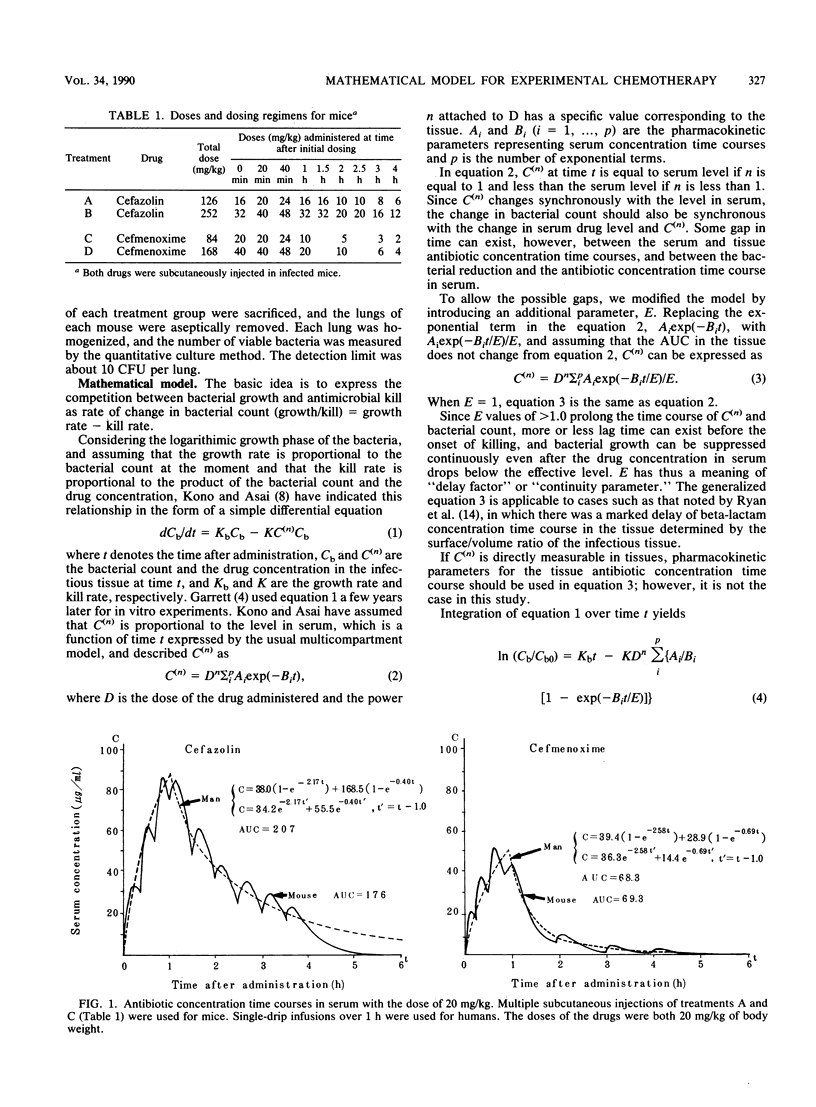
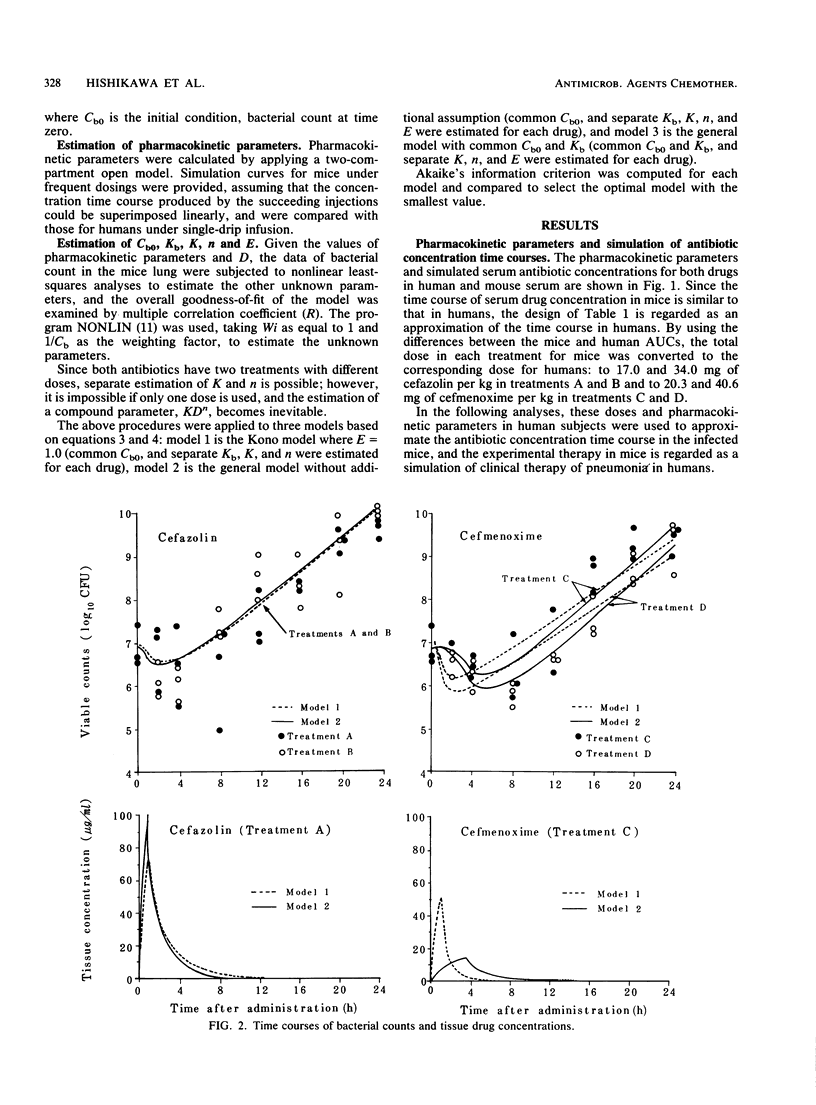
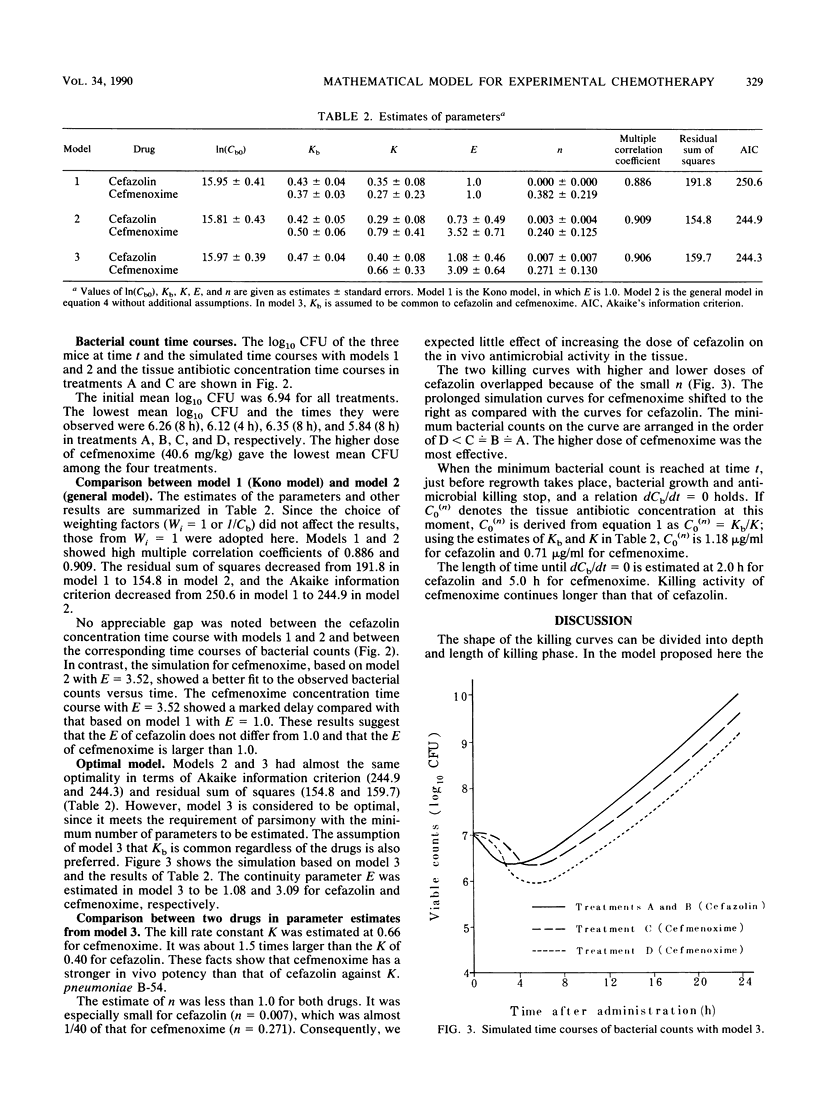
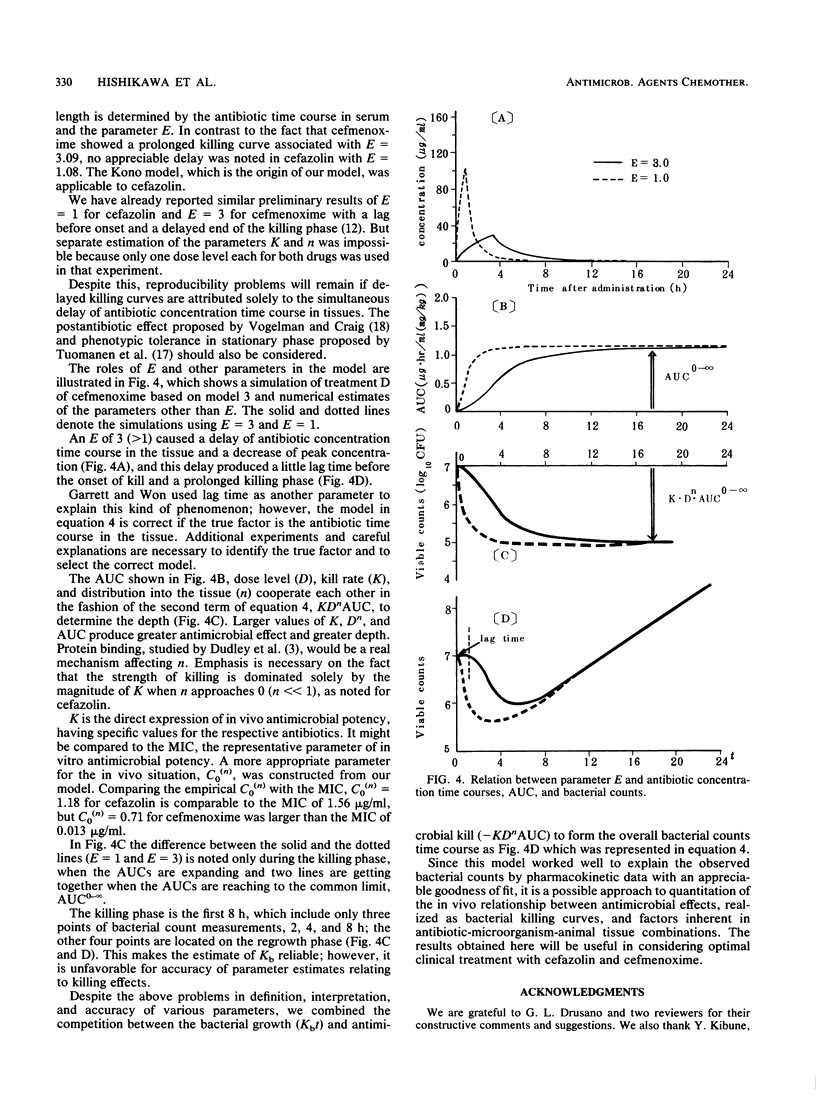
Abstract
Two beta-lactam antibiotics, cefazolin and cefmenoxime, were administered in an experimental model of murine pneumonia caused by Klebsiella pneumoniae in a way which enabled us to approximate the serum antibiotic concentration time course in humans. Bacterial counts during the experiments were subjected to nonlinear least-squares analyses by using a mathematical model that explained the bacterial killing by the antibiotic concentration time course and other factors associated with antimicrobial potency and bacterial growth. Cefazolin gave a killing curve that changed synchronously with the drug levels in serum; in contrast, cefmenoxime gave a curve that was prolonged as compared with the change in the drug levels in serum. Multiple correlation coefficients were about 0.9, and the model worked well for bacterial count data. Parameters relating to antimicrobial potency of the drugs, bacterial growth rate, and drug distribution into the tissue were estimated numerically.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T., Engeset A., Olszewski W., Ostby N., Solberg R. Extravascular penetration of highly protein-bound flucloxacillin. Antimicrob Agents Chemother. 1986 Nov;30(5):729–732. doi: 10.1128/aac.30.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M. N., Shyu W. C., Nightingale C. H., Quintiliani R. Effect of saturable serum protein binding on the pharmacokinetics of unbound cefonicid in humans. Antimicrob Agents Chemother. 1986 Oct;30(4):565–569. doi: 10.1128/aac.30.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. R., Won C. M. Kinetics and mechanisms of drug action on microorganisms. XVII. Bactericidal effects of penicillin, kanamycin, and rifampin with and without organism pretreatment with bacteriostatic chloramphenicol, tetracycline, and novobiocin. J Pharm Sci. 1973 Oct;62(10):1666–1673. doi: 10.1002/jps.2600621018. [DOI] [PubMed] [Google Scholar]
- Grasso S., Meinardi G., de Carneri I., Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother. 1978 Apr;13(4):570–576. doi: 10.1128/aac.13.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano S., Tsuji A., Asano T., Tamai I., Nakashima E., Yamana T., Mitsuhashi S. Kinetic analysis and characterization of the bacterial regrowth after treatment of Escherichia coli with beta-lactam antibiotics. J Pharm Sci. 1984 Oct;73(10):1422–1427. doi: 10.1002/jps.2600731025. [DOI] [PubMed] [Google Scholar]
- Kita Y., Fugono T., Imada A. Comparative pharmacokinetics of carumonam and aztreonam in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob Agents Chemother. 1986 Jan;29(1):127–134. doi: 10.1128/aac.29.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T., Asai T. Kinetics of fermentation processes. Biotechnol Bioeng. 1969 May;11(3):293–321. doi: 10.1002/bit.260110304. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uzuka Y., Nagatake T., Shishido H., Suzuki H., Noguchi Y., Tamaki K., Rah S., Ide M. [Experimental pneumonia due to gram negative bacilli by air-borne infection (author's transl)]. Nihon Kyobu Shikkan Gakkai Zasshi. 1978 Aug;16(8):581–588. [PubMed] [Google Scholar]
- Nishi T., Tsuchiya K. Experimental respiratory tract infection with Klebsiella pneumoniae DT-S in mice: chemotherapy with kanamycin. Antimicrob Agents Chemother. 1980 Mar;17(3):494–505. doi: 10.1128/aac.17.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. M., Cars O., Hoffstedt B. The use of antibiotic serum levels to predict concentrations in tissues. Scand J Infect Dis. 1986;18(5):381–388. doi: 10.3109/00365548609032352. [DOI] [PubMed] [Google Scholar]
- Shibl A. M., Hackbarth C. J., Sande M. A. Evaluation of pefloxacin in experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1986 Mar;29(3):409–411. doi: 10.1128/aac.29.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji A., Hamano S., Asano T., Nakashima E., Yamana T., Mitsuhashi S. Microbial kinetics of beta-lactam antibiotics against Escherichia coli. J Pharm Sci. 1984 Oct;73(10):1418–1422. doi: 10.1002/jps.2600731024. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Durack D. T., Tomasz A. Antibiotic tolerance among clinical isolates of bacteria. Antimicrob Agents Chemother. 1986 Oct;30(4):521–527. doi: 10.1128/aac.30.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelman B. S., Craig W. A. Postantibiotic effects. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):37–46. doi: 10.1093/jac/15.suppl_a.37. [DOI] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Cadge B., Durham S. R., Baker S. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J Infect Dis. 1980 Jul;142(1):77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]


