Abstract
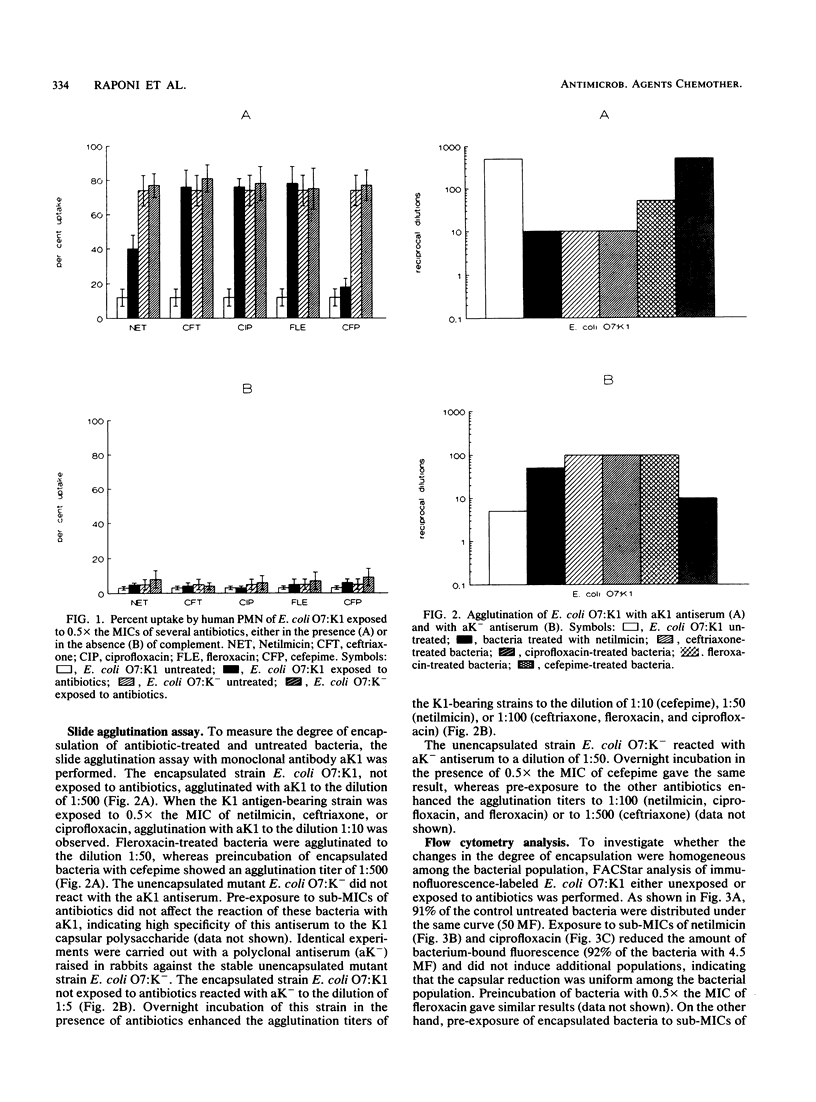
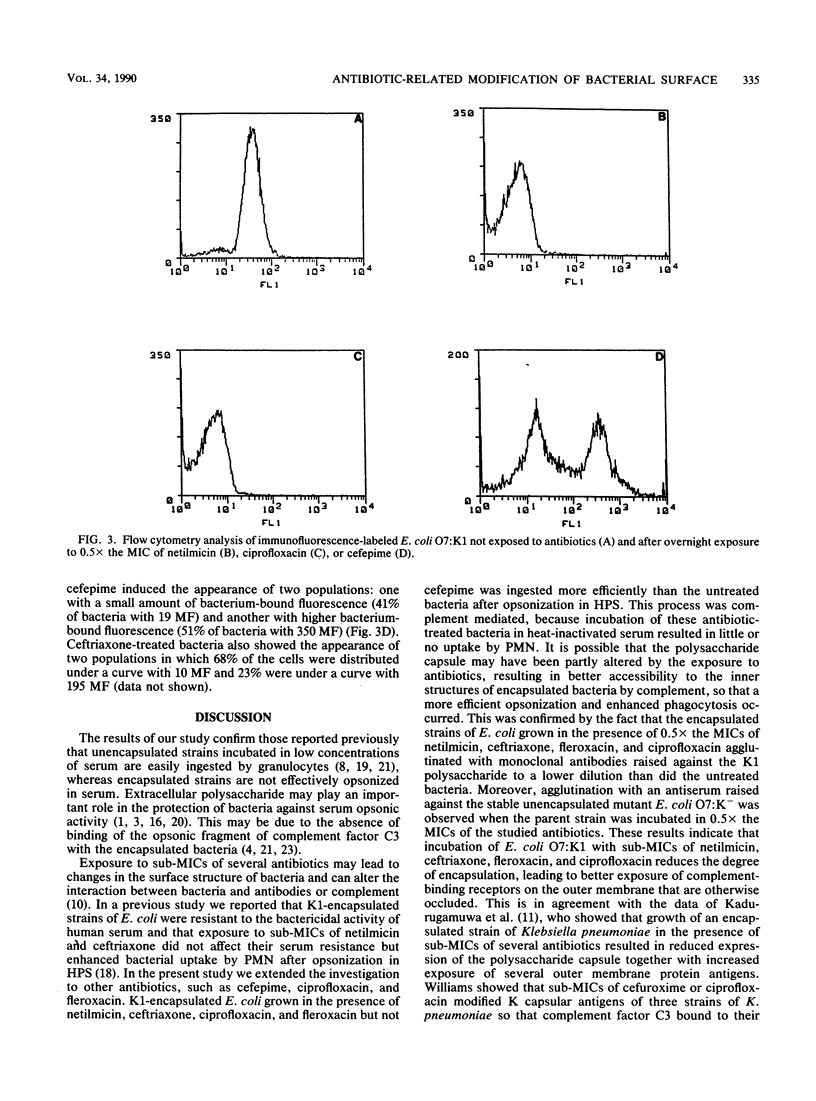
The influence of five antibiotics (netilmicin, ceftriaxone, cefepime, fleroxacin, and ciprofloxacin) on capsular polysaccharide distribution and on opsonophagocytosis by human polymorphonuclear leukocytes of unencapsulated and encapsulated Escherichia coli strains was studied. Unencapsulated E. coli strains were readily opsonized in serum and easily ingested by polymorphonuclear leukocytes, and antibiotics did not further enhance the phagocytosis rates. In contrast, encapsulated bacteria were poorly opsonized in human serum, and phagocytosis was enhanced after overnight exposure to 0.5x the MICs of the antibiotics, with the exception of cefepime. Incubation of unencapsulated as well as encapsulated bacteria in complement-inactivated serum markedly reduced the bacterial uptake by polymorphonuclear leukocytes regardless of the presence of antibiotics. Slide agglutination assays, performed either with a monoclonal antibody for capsular polysaccharide or with an antiserum raised against the stable unencapsulated mutant E. coli O7:K-, showed reduction but not lack of the capsular polysaccharide of encapsulated E. coli O7:K1, and better exposure of subcapsular epitopes, after incubation with 0.5x the MICs of antibiotics. Flow cytometric analysis of encapsulated E. coli exposed to netilmicin, ciprofloxacin, and fleroxacin revealed that the reduction in capsular material was homogeneous among the bacterial population. Treatment with cefepime and ceftriaxone induced two populations of bacteria that differed in the amount of K antigen present. These results indicate that sub-MICs of netilmicin, ceftriaxone, fleroxacin, and ciprofloxacin influenced complement-mediated opsonization, probably due to changes in the capsular polysaccharide structure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Gemski P., Sadoff J. C., Orskov F., Orskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis. 1984 Feb;149(2):184–193. doi: 10.1093/infdis/149.2.184. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Kim K. S., Wright D. C., Sadoff J. C., Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis. 1986 Sep;154(3):497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- Emslie K. R., Nade S. Pathogenesis and treatment of acute hematogenous osteomyelitis: evaluation of current views with reference to an animal model. Rev Infect Dis. 1986 Nov-Dec;8(6):841–849. doi: 10.1093/clinids/8.6.841. [DOI] [PubMed] [Google Scholar]
- Gemmell C. G. Changes in expression of bacterial surface antigens induced by antibiotics and their influence on host defenses. Pathol Biol (Paris) 1987 Dec;35(10 Pt 2):1377–1381. [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Zak O. Effect of subinhibitory concentrations of cephalosporins on surface properties and siderophore production in iron-depleted Klebsiella pneumoniae. Antimicrob Agents Chemother. 1985 Feb;27(2):220–223. doi: 10.1128/aac.27.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Zak O. Protein antigens of encapsulated Klebsiella pneumoniae surface exposed after growth in the presence of subinhibitory concentrations of cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):195–199. doi: 10.1128/aac.28.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Schneider H., Brandt B. L. Production and degradation of serogroup B Neisseria meningitidis polysaccharide. Infect Immun. 1972 Nov;6(5):657–658. doi: 10.1128/iai.6.5.657-661.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R., Carling P. C., Bruins S., Greely A. The relation of K-antigen to virulence of Escherichia coli. J Infect Dis. 1975 Jan;131(1):6–10. doi: 10.1093/infdis/131.1.6. [DOI] [PubMed] [Google Scholar]
- Milatovic D. Antibiotics and phagocytosis. Eur J Clin Microbiol. 1983 Oct;2(5):414–425. doi: 10.1007/BF02013898. [DOI] [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Phillips A. P., Martin K. L., Capey A. J. Direct and indirect immunofluorescence analysis of bacterial populations by flow cytometry. J Immunol Methods. 1987 Aug 3;101(2):219–228. doi: 10.1016/0022-1759(87)90153-0. [DOI] [PubMed] [Google Scholar]
- Raponi G., Vreede R. W., Rozenberg-Arska M., Hoepelman I. M., Keller N., Verhoef J. The influence of subminimal inhibitory concentrations of netilmicin and ceftriaxone on the interaction of Escherichia coli with host defences. J Antimicrob Chemother. 1989 Apr;23(4):565–576. doi: 10.1093/jac/23.4.565. [DOI] [PubMed] [Google Scholar]
- Rozenberg-Arska M., Porsius J. C., Jaarsma E. Y., Verhoef J. Bactericidal, bacteriolytic and opsonic activity of human serum against Escherichia coli. J Med Microbiol. 1986 Sep;22(2):143–149. doi: 10.1099/00222615-22-2-143. [DOI] [PubMed] [Google Scholar]
- Rozenberg-Arska M., Salters M. E., van Strijp J. A., Geuze J. J., Verhoef J. Electron microscopic study of phagocytosis of Escherichia coli by human polymorphonuclear leukocytes. Infect Immun. 1985 Dec;50(3):852–859. doi: 10.1128/iai.50.3.852-859.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Peters R., Peterson P. K., Verhoef J. Phagocytosis and killing of staphylococci by human polymorphonuclear and mononuclear leucocytes. J Clin Pathol. 1978 Jun;31(6):539–545. doi: 10.1136/jcp.31.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., van Erne M. E., Peters R., Peterson P. K., Verhoef J. Quantitation of the third component of human complement attached to the surface of opsonized bacteria: opsonin-deficient sera and phagocytosis-resistant strains. Infect Immun. 1979 Dec;26(3):808–814. doi: 10.1128/iai.26.3.808-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Williams P. Sub-MICs of cefuroxime and ciprofloxacin influence interaction of complement and immunoglobulins with Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987 May;31(5):758–762. doi: 10.1128/aac.31.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]


