Abstract
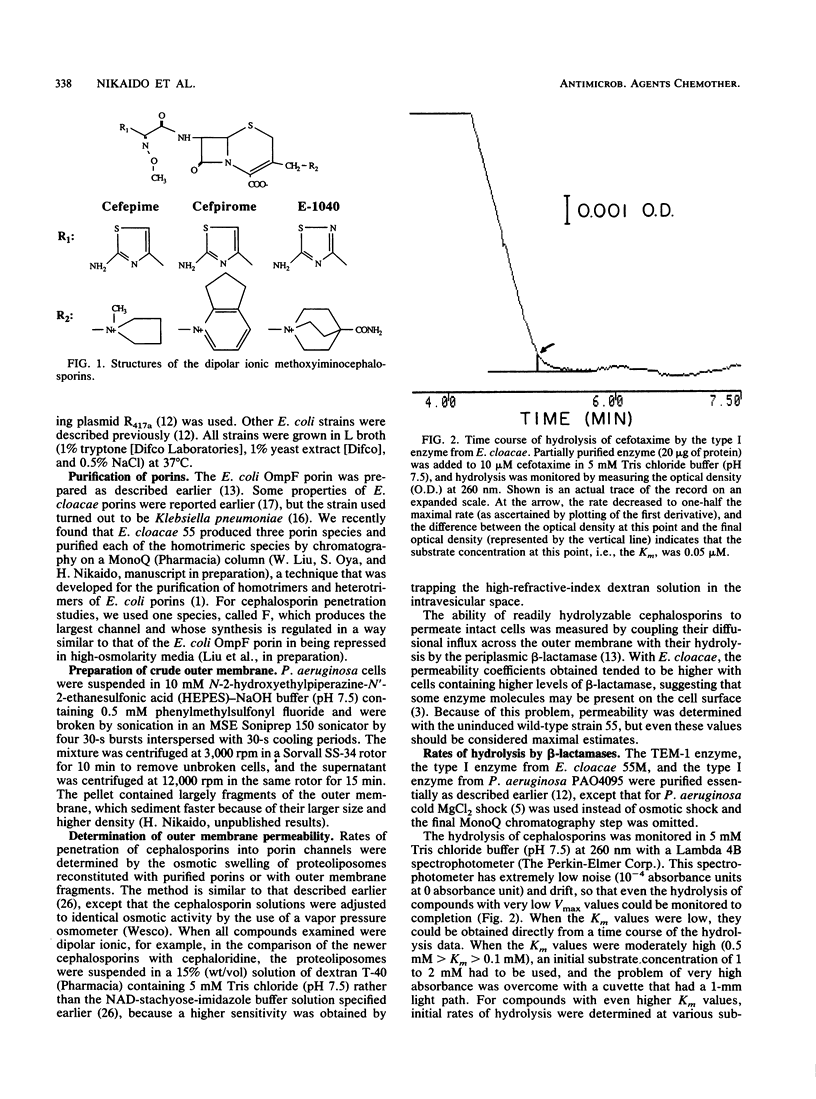
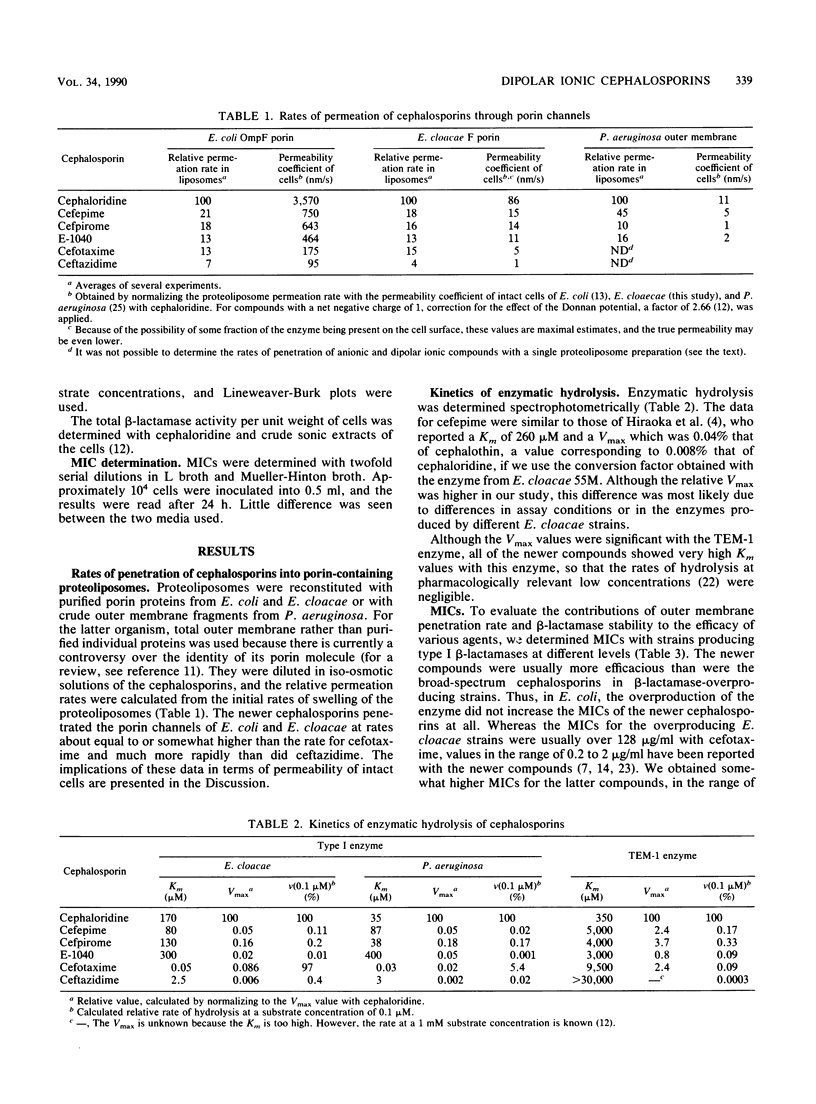
Some enteric bacteria, such as Enterobacter cloacae, can develop high-level resistance to broad-spectrum cephalosporins by overproducing their chromosomally encoded type I beta-lactamases. This is because these agents are hydrolyzed rapidly at pharmacologically relevant, low (0.1 to 1 microM), concentrations, owing to their high affinity for type I enzymes. In contrast, the more recently developed cephalosporins, with quaternary-nitrogen-containing substituents at the 3 position, show increased efficacy against beta-lactamase-overproducing strains and, indeed, have a much lower affinity for type I enzymes. However, the possible contribution of an improved outer membrane permeability in their increased efficacy has not been studied. We found by proteoliposome swelling assays that cefepime, cefpirome, and E-1040 all penetrated the porin channels of Escherichia coli and E. cloacae much more rapidly than did ceftazidime and at least as rapidly as did cefotaxime. Considering that the influx of anionic compounds such as cefotaxime and ceftazidime will be further retarded in intact cells, owing to the Donnan potential, we expect that the newer compounds will penetrate intact cells 2 to 10 times more rapidly than will cefotaxime and ceftazidime. The kinetic parameters of hydrolysis of these agents by E. cloacae beta-lactamase showed that at 0.1 microM, they were hydrolyzed much more slowly than was cefotaxime and at about the same rate as or a lower rate than was ceftazidime. The combination of these two effects explains nearly quantitatively why these newer agents are more effective against some of the beta-lactamase-overproducing gram-negative bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gehring K. B., Nikaido H. Existence and purification of porin heterotrimers of Escherichia coli K12 OmpC, OmpF, and PhoE proteins. J Biol Chem. 1989 Feb 15;264(5):2810–2815. [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C. Characterization of beta-lactamase induction in Enterobacter cloacae. Antimicrob Agents Chemother. 1983 Jan;23(1):91–97. doi: 10.1128/aac.23.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewinson R. G., Cartwright S. J., Slack M. P., Whipp R. D., Woodward M. J., Nichols W. W. Permeability to cefsulodin of the outer membrane of Pseudomonas aeruginosa and discrimination between beta-lactamase-mediated trapping and hydrolysis as mechanisms of resistance. Eur J Biochem. 1989 Feb 15;179(3):667–675. doi: 10.1111/j.1432-1033.1989.tb14599.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka M., Masuyoshi S., Mitsuhashi S., Tomatsu K., Inoue M. Cephalosporinase interactions and antimicrobial activity of BMY-28142, ceftazidime and cefotaxime. J Antibiot (Tokyo) 1988 Jan;41(1):86–93. doi: 10.7164/antibiotics.41.86. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Kageyama M. Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa. J Bacteriol. 1980 Mar;141(3):1055–1063. doi: 10.1128/jb.141.3.1055-1063.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., De Meester F., Galleni M., Masson S., Dusart J., Frère J. M., Van Beeumen J., Bush K., Sykes R. Properties of a class C beta-lactamase from Serratia marcescens. Biochem J. 1986 Nov 1;239(3):581–586. doi: 10.1042/bj2390581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Arai S., Hayashi S., Fujimoto K. Beta-lactamase stability of cefpirome (HR 810), a new cephalosporin with a broad antimicrobial spectrum. Antimicrob Agents Chemother. 1986 Nov;30(5):713–718. doi: 10.1128/aac.30.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M. Do beta-lactamases 'trap' cephalosporins? J Antimicrob Chemother. 1985 May;15(5):511–514. doi: 10.1093/jac/15.5.511. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Kinetics and significance of the activity of the Sabath and Abrahams' beta-lactamase of Pseudomonas aeruginosa against cefotaxime and cefsulodin. J Antimicrob Chemother. 1983 Feb;11(2):169–179. doi: 10.1093/jac/11.2.169. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 1985;27(2):197–231. doi: 10.1016/0163-7258(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. J., Carlton D. D., Farrell C. A., Kessler R. E. Affinity of cephalosporins for beta-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986 May;29(5):845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis. 1983 Mar;147(3):585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Hellman J., Nikaido H. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J Biol Chem. 1988 Jan 25;263(3):1182–1187. [PubMed] [Google Scholar]
- Takahashi I., Sawai T., Ando T., Yamagishi S. Cefoxitin resistance by a chromosomal cephalosporinase in Escherichia coli. J Antibiot (Tokyo) 1980 Sep;33(9):1037–1042. doi: 10.7164/antibiotics.33.1037. [DOI] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Katsu K., Moriyama M., Kitoh K. In vitro evaluation of E1040, a new cephalosporin with potent antipseudomonal activity. Antimicrob Agents Chemother. 1988 May;32(5):693–701. doi: 10.1128/aac.32.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


