Abstract
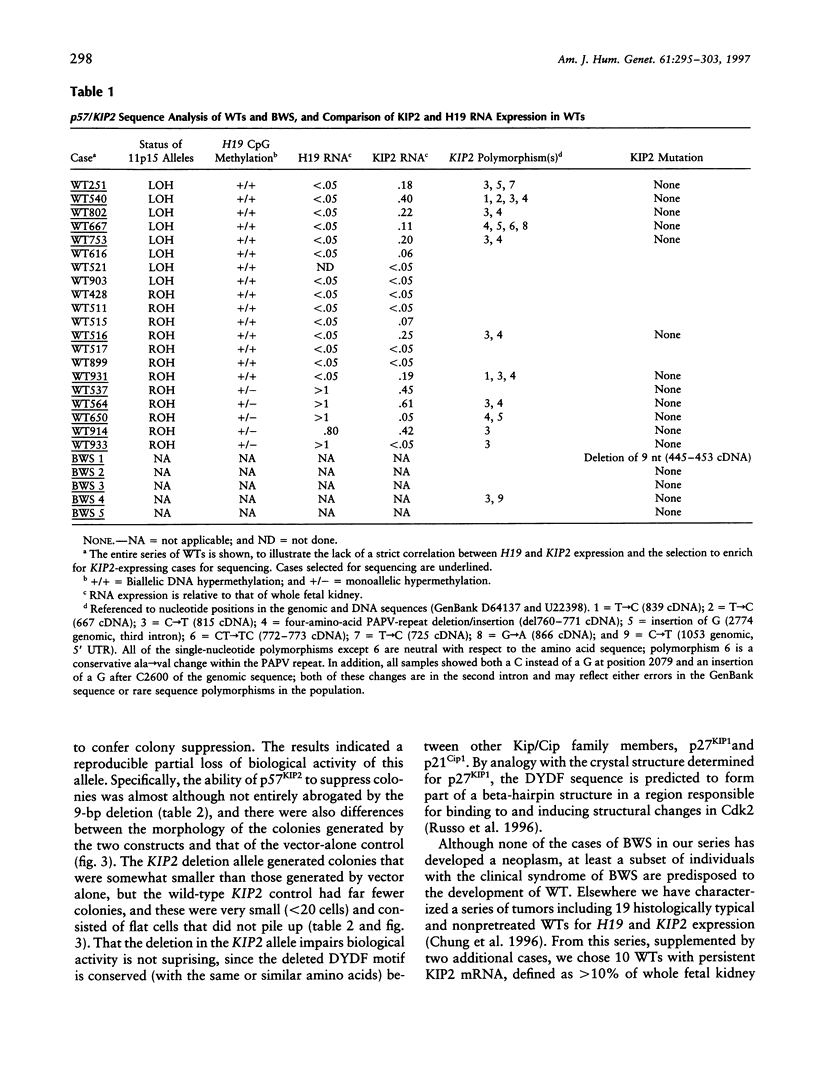
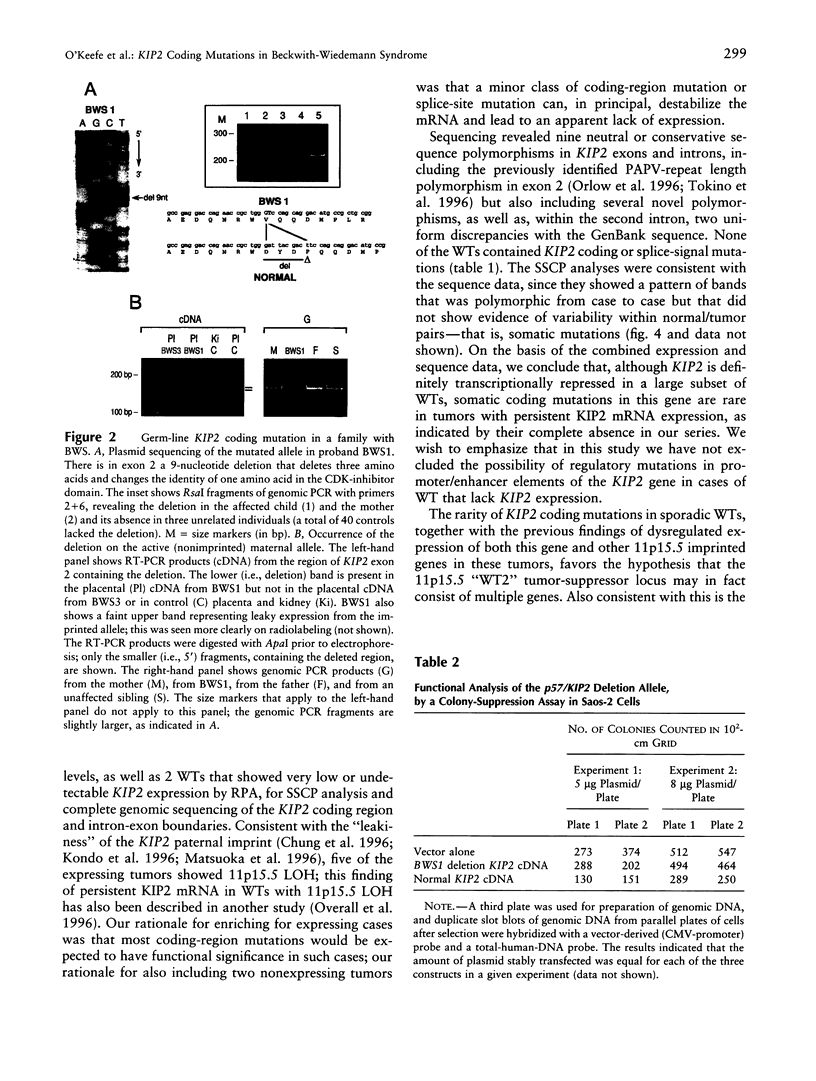
The Beckwith-Wiedemann syndrome (BWS) is marked by fetal organ overgrowth and conveys a predisposition to certain childhood tumors, including Wilms tumor (WT). The genetics of BWS have implicated a gene that maps to chromosome 11p15 and is paternally imprinted, and the gene encoding the cyclin-cdk inhibitor p57KIP2 has been a strong candidate. By complete sequencing of the coding exons and intron/exon junctions, we found a maternally transmitted coding mutation in the cdk-inhibitor domain of the KIP2 gene in one of five cases of BWS. The BWS mutation was an in-frame three-amino-acid deletion that significantly reduced but did not fully abrogate growth-suppressive activity in a transfection assay. In contrast, no somatic coding mutations in KIP2 were found in a set of 12 primary WTs enriched for cases that expressed KIP2 mRNA, including cases with and without 11p15.5 loss of heterozygosity. Two other 11p15.5 loci, the linked and oppositely imprinted H19 and IGF2 genes, have been previously implicated in WT pathogenesis, and several of the tumors with persistent KIP2 mRNA expression and absence of KIP2 coding mutations showed full inactivation of H19. These data suggest that KIP2 is a BWS gene but that it is not uniquely equivalent to the 11p15.5 "WT2" tumor-suppressor locus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chung W. Y., Yuan L., Feng L., Hensle T., Tycko B. Chromosome 11p15.5 regional imprinting: comparative analysis of KIP2 and H19 in human tissues and Wilms' tumors. Hum Mol Genet. 1996 Aug;5(8):1101–1108. doi: 10.1093/hmg/5.8.1101. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Hatada I., Inazawa J., Abe T., Nakayama M., Kaneko Y., Jinno Y., Niikawa N., Ohashi H., Fukushima Y., Iida K. Genomic imprinting of human p57KIP2 and its reduced expression in Wilms' tumors. Hum Mol Genet. 1996 Jun;5(6):783–788. doi: 10.1093/hmg/5.6.783. [DOI] [PubMed] [Google Scholar]
- Hatada I., Mukai T. Genomic imprinting of p57KIP2, a cyclin-dependent kinase inhibitor, in mouse. Nat Genet. 1995 Oct;11(2):204–206. doi: 10.1038/ng1095-204. [DOI] [PubMed] [Google Scholar]
- Hatada I., Ohashi H., Fukushima Y., Kaneko Y., Inoue M., Komoto Y., Okada A., Ohishi S., Nabetani A., Morisaki H. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996 Oct;14(2):171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- Henry I., Bonaiti-Pellié C., Chehensse V., Beldjord C., Schwartz C., Utermann G., Junien C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature. 1991 Jun 20;351(6328):665–667. doi: 10.1038/351665a0. [DOI] [PubMed] [Google Scholar]
- Henry I., Puech A., Riesewijk A., Ahnine L., Mannens M., Beldjord C., Bitoun P., Tournade M. F., Landrieu P., Junien C. Somatic mosaicism for partial paternal isodisomy in Wiedemann-Beckwith syndrome: a post-fertilization event. Eur J Hum Genet. 1993;1(1):19–29. doi: 10.1159/000472384. [DOI] [PubMed] [Google Scholar]
- Hoovers J. M., Kalikin L. M., Johnson L. A., Alders M., Redeker B., Law D. J., Bliek J., Steenman M., Benedict M., Wiegant J. Multiple genetic loci within 11p15 defined by Beckwith-Wiedemann syndrome rearrangement breakpoints and subchromosomal transferable fragments. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12456–12460. doi: 10.1073/pnas.92.26.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junien C. Beckwith-Wiedemann syndrome, tumourigenesis and imprinting. Curr Opin Genet Dev. 1992 Jun;2(3):431–438. doi: 10.1016/s0959-437x(05)80154-6. [DOI] [PubMed] [Google Scholar]
- Kondo M., Matsuoka S., Uchida K., Osada H., Nagatake M., Takagi K., Harper J. W., Takahashi T., Elledge S. J., Takahashi T. Selective maternal-allele loss in human lung cancers of the maternally expressed p57KIP2 gene at 11p15.5. Oncogene. 1996 Mar 21;12(6):1365–1368. [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Leighton P. A., Ingram R. S., Eggenschwiler J., Efstratiadis A., Tilghman S. M. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995 May 4;375(6526):34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- Mannens M., Hoovers J. M., Redeker E., Verjaal M., Feinberg A. P., Little P., Boavida M., Coad N., Steenman M., Bliek J. Parental imprinting of human chromosome region 11p15.3-pter involved in the Beckwith-Wiedemann syndrome and various human neoplasia. Eur J Hum Genet. 1994;2(1):3–23. doi: 10.1159/000472337. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Edwards M. C., Bai C., Parker S., Zhang P., Baldini A., Harper J. W., Elledge S. J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995 Mar 15;9(6):650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Thompson J. S., Edwards M. C., Bartletta J. M., Grundy P., Kalikin L. M., Harper J. W., Elledge S. J., Feinberg A. P. Imprinting of the gene encoding a human cyclin-dependent kinase inhibitor, p57KIP2, on chromosome 11p15. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):3026–3030. doi: 10.1073/pnas.93.7.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison I. M., Becroft D. M., Taniguchi T., Woods C. G., Reeve A. E. Somatic overgrowth associated with overexpression of insulin-like growth factor II. Nat Med. 1996 Mar;2(3):311–316. doi: 10.1038/nm0396-311. [DOI] [PubMed] [Google Scholar]
- Moulton T., Chung W. Y., Yuan L., Hensle T., Waber P., Nisen P., Tycko B. Genomic imprinting and Wilms' tumor. Med Pediatr Oncol. 1996 Nov;27(5):476–483. doi: 10.1002/(SICI)1096-911X(199611)27:5<476::AID-MPO15>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Moulton T., Crenshaw T., Hao Y., Moosikasuwan J., Lin N., Dembitzer F., Hensle T., Weiss L., McMorrow L., Loew T. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994 Jul;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- Orlow I., Iavarone A., Crider-Miller S. J., Bonilla F., Latres E., Lee M. H., Gerald W. L., Massagué J., Weissman B. E., Cordón-Cardó C. Cyclin-dependent kinase inhibitor p57KIP2 in soft tissue sarcomas and Wilms'tumors. Cancer Res. 1996 Mar 15;56(6):1219–1221. [PubMed] [Google Scholar]
- Overall M. L., Spencer J., Bakker M., Dziadek M., Smith P. J. p57K1P2 is expressed in Wilms' tumor with LOH of 11p15.5. Genes Chromosomes Cancer. 1996 Sep;17(1):56–59. doi: 10.1002/(SICI)1098-2264(199609)17:1<56::AID-GCC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ping A. J., Reeve A. E., Law D. J., Young M. R., Boehnke M., Feinberg A. P. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989 May;44(5):720–723. [PMC free article] [PubMed] [Google Scholar]
- Rainier S., Johnson L. A., Dobry C. J., Ping A. J., Grundy P. E., Feinberg A. P. Relaxation of imprinted genes in human cancer. Nature. 1993 Apr 22;362(6422):747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- Reik W., Brown K. W., Schneid H., Le Bouc Y., Bickmore W., Maher E. R. Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by altered imprinting pattern in the IGF2-H19 domain. Hum Mol Genet. 1995 Dec;4(12):2379–2385. doi: 10.1093/hmg/4.12.2379. [DOI] [PubMed] [Google Scholar]
- Russo A. A., Jeffrey P. D., Patten A. K., Massagué J., Pavletich N. P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996 Jul 25;382(6589):325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- Steenman M. J., Rainier S., Dobry C. J., Grundy P., Horon I. L., Feinberg A. P. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet. 1994 Jul;7(3):433–439. doi: 10.1038/ng0794-433. [DOI] [PubMed] [Google Scholar]
- Tokino T., Urano T., Furuhata T., Matsushima M., Miyatsu T., Sasaki S., Nakamura Y. Characterization of the human p57KIP2 gene: alternative splicing, insertion/deletion polymorphisms in VNTR sequences in the coding region, and mutational analysis. Hum Genet. 1996 May;97(5):625–631. doi: 10.1007/BF02281873. [DOI] [PubMed] [Google Scholar]
- Tycko B., Feng L., Nguyen L., Francis A., Hays A., Chung W. Y., Tang M. X., Stern Y., Sahota A., Hendrie H. Polymorphisms in the human apolipoprotein-J/clusterin gene: ethnic variation and distribution in Alzheimer's disease. Hum Genet. 1996 Oct;98(4):430–436. doi: 10.1007/s004390050234. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Shen D. R., Fei Y. L., Song Q. L., Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993 Oct;5(2):143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Squire J. A. Molecular biology of Beckwith-Wiedemann syndrome. Med Pediatr Oncol. 1996 Nov;27(5):462–469. doi: 10.1002/(SICI)1096-911X(199611)27:5<462::AID-MPO13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]