Abstract
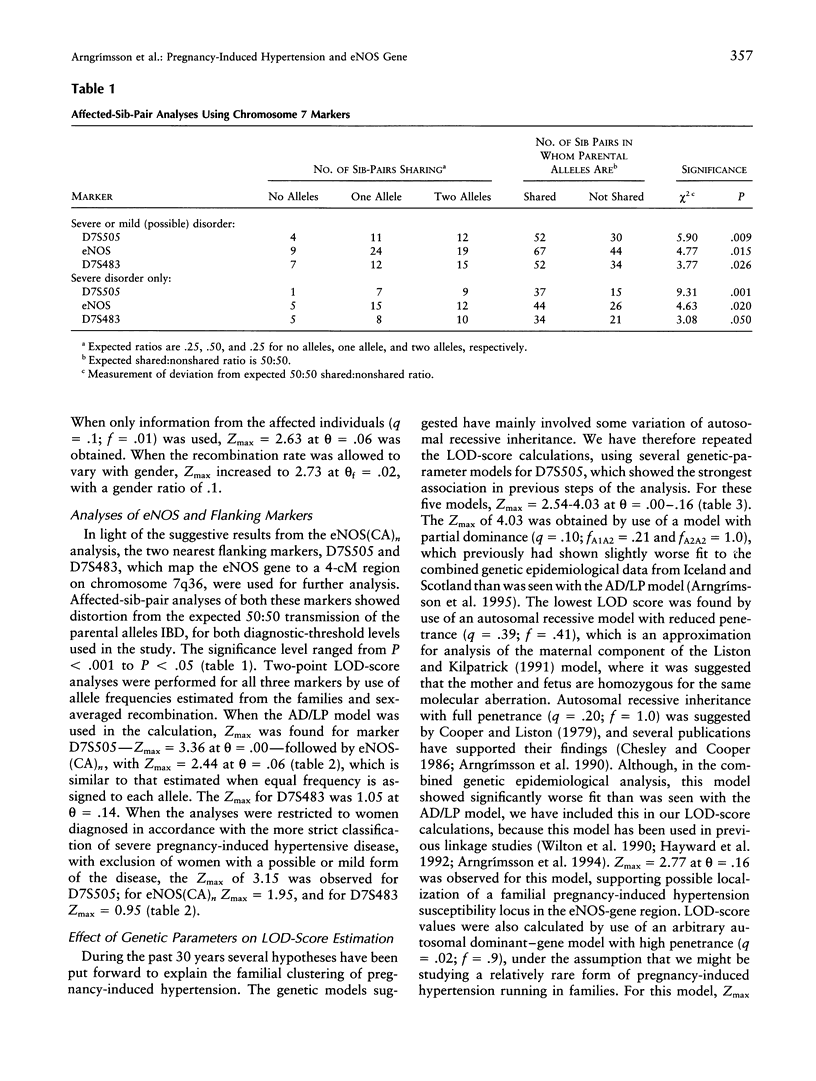
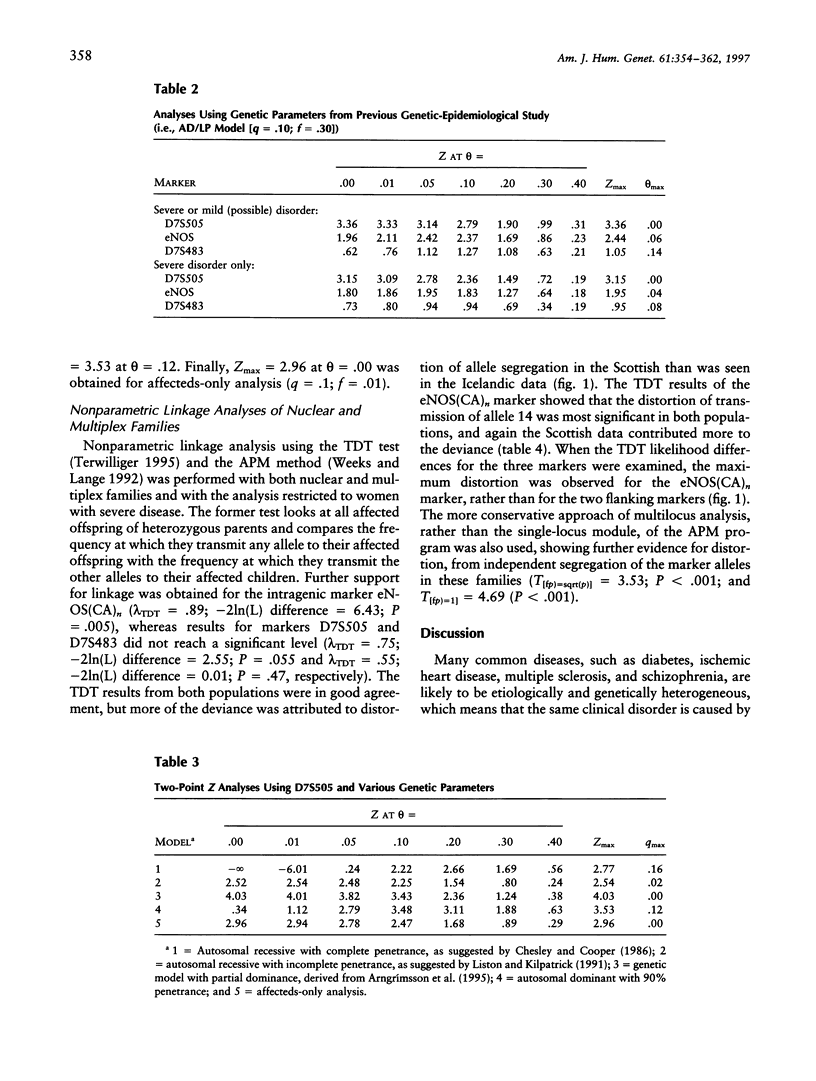
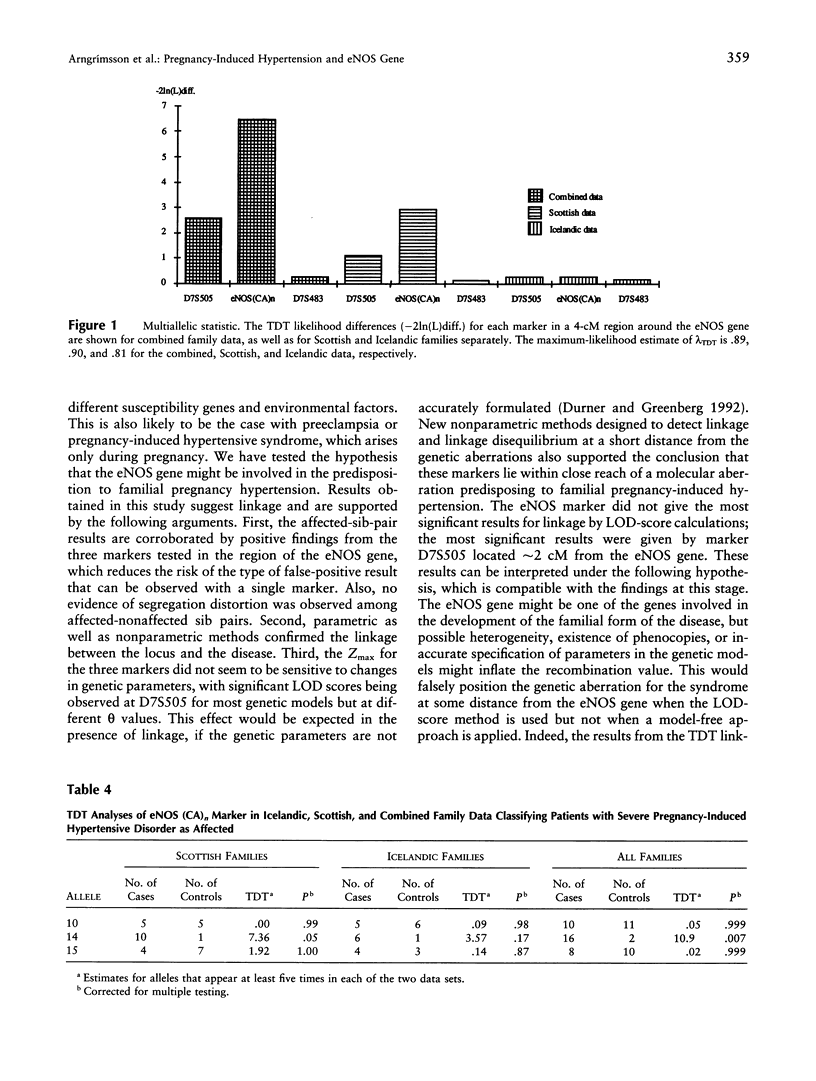
Pregnancy-induced hypertension may be regarded as a manifestation of endothelial-cell dysfunction. The role of the eNOS gene in the development of a familial pregnancy-induced hypertension was evaluated by analysis of linkage among affected sisters and in multiplex families (n = 50). Markers from a 4-cM region encoding the eNOS gene showed distortion from the expected allele sharing among affected sisters (P = .001-.05), and the statistic obtained from the multilocus application of the affected-pedigree-member method also showed distortion (T[f(P)=sqrt(P)] = 3.53; P < .001). A LOD score of 3.36 was obtained for D7S505 when a best-fitting model derived from genetic epidemiological data was used, and LOD scores of 2.54-4.03 were obtained when various other genetic models were used. Estimates of recombination rate, rather than maximum LOD-score values, were affected by changes in the genetic parameters. The transmission-disequilibrium test, a model-free estimate of linkage, showed strongest association and linkage with a microsatellite within intron 13 of the eNOS gene (P = .005). These results support the localization of a familial pregnancy-induced hypertension-susceptibility locus in the region of chromosome 7q36 encoding the eNOS gene.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arngrimsson R., Björnsson S., Geirsson R. T., Björnsson H., Walker J. J., Snaedal G. Genetic and familial predisposition to eclampsia and pre-eclampsia in a defined population. Br J Obstet Gynaecol. 1990 Sep;97(9):762–769. doi: 10.1111/j.1471-0528.1990.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Arngrímsson R., Geirsson R. T., Cooke A., Connor M., Björnsson S., Walker J. J. Renin gene restriction fragment length polymorphisms do not show linkage with preeclampsia and eclampsia. Acta Obstet Gynecol Scand. 1994 Jan;73(1):10–13. doi: 10.3109/00016349409013385. [DOI] [PubMed] [Google Scholar]
- Arngrímsson R., Purandare S., Connor M., Walker J. J., Björnsson S., Soubrier F., Kotelevtsev Y. V., Geirsson R. T., Björnsson H. Angiotensinogen: a candidate gene involved in preeclampsia? Nat Genet. 1993 Jun;4(2):114–115. doi: 10.1038/ng0693-114. [DOI] [PubMed] [Google Scholar]
- BROWNE F. J., SHEUMACK D. R. Chronic hypertension following pre-eclamptic toxaemia; the influence of familial hypertension on its causation. J Obstet Gynaecol Br Emp. 1956 Oct;63(5):677–679. doi: 10.1111/j.1471-0528.1956.tb05545.x. [DOI] [PubMed] [Google Scholar]
- Baker P. N., Davidge S. T., Roberts J. M. Plasma from women with preeclampsia increases endothelial cell nitric oxide production. Hypertension. 1995 Aug;26(2):244–248. doi: 10.1161/01.hyp.26.2.244. [DOI] [PubMed] [Google Scholar]
- Blackwelder W. C., Elston R. C. A comparison of sib-pair linkage tests for disease susceptibility loci. Genet Epidemiol. 1985;2(1):85–97. doi: 10.1002/gepi.1370020109. [DOI] [PubMed] [Google Scholar]
- Cooper D. W., Liston W. A. Genetic control of severe pre-eclampsia. J Med Genet. 1979 Dec;16(6):409–416. doi: 10.1136/jmg.16.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottingham R. W., Jr, Idury R. M., Schäffer A. A. Faster sequential genetic linkage computations. Am J Hum Genet. 1993 Jul;53(1):252–263. [PMC free article] [PubMed] [Google Scholar]
- Delacrétaz E., de Quay N., Waeber B., Vial Y., Schulz P. E., Burnier M., Brunner H. R., Bossart H., Schaad N. C. Differential nitric oxide synthase activity in human platelets during normal pregnancy and pre-eclampsia. Clin Sci (Lond) 1995 Jun;88(6):607–610. doi: 10.1042/cs0880607. [DOI] [PubMed] [Google Scholar]
- Durner M., Greenberg D. A. Effect of heterogeneity and assumed mode of inheritance on lod scores. Am J Med Genet. 1992 Feb 1;42(3):271–275. doi: 10.1002/ajmg.1320420302. [DOI] [PubMed] [Google Scholar]
- Gant N. F., Daley G. L., Chand S., Whalley P. J., MacDonald P. C. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973 Nov;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz R. M., Morano I., Calovini T., Studer R., Holtz J. Increased expression of endothelial constitutive nitric oxide synthase in rat aorta during pregnancy. Biochem Biophys Res Commun. 1994 Nov 30;205(1):905–910. doi: 10.1006/bbrc.1994.2750. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Davies R. L., Harrison T. J., Evans K. T. EDRF coordinates the behaviour of vascular resistance vessels. Nature. 1987 Oct 1;329(6138):442–445. doi: 10.1038/329442a0. [DOI] [PubMed] [Google Scholar]
- Groenendijk R., Trimbos J. B., Wallenburg H. C. Hemodynamic measurements in preeclampsia: preliminary observations. Am J Obstet Gynecol. 1984 Oct 1;150(3):232–236. doi: 10.1016/s0002-9378(84)90357-0. [DOI] [PubMed] [Google Scholar]
- Hayward C., Livingstone J., Holloway S., Liston W. A., Brock D. J. An exclusion map for pre-eclampsia: assuming autosomal recessive inheritance. Am J Hum Genet. 1992 Apr;50(4):749–757. [PMC free article] [PubMed] [Google Scholar]
- Huang P. L., Huang Z., Mashimo H., Bloch K. D., Moskowitz M. A., Bevan J. A., Fishman M. C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995 Sep 21;377(6546):239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- KAKU M., NAGATA H. Hypertensive lineage and toxemia of pregnancy. Am J Obstet Gynecol. 1959 Aug;78(2):399–404. doi: 10.1016/0002-9378(59)90191-7. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston W. A., Kilpatrick D. C. Is genetic susceptibility to pre-eclampsia conferred by homozygosity for the same single recessive gene in mother and fetus? Br J Obstet Gynaecol. 1991 Nov;98(11):1079–1086. doi: 10.1111/j.1471-0528.1991.tb15358.x. [DOI] [PubMed] [Google Scholar]
- McCarthy A. L., Woolfson R. G., Raju S. K., Poston L. Abnormal endothelial cell function of resistance arteries from women with preeclampsia. Am J Obstet Gynecol. 1993 Apr;168(4):1323–1330. doi: 10.1016/0002-9378(93)90389-z. [DOI] [PubMed] [Google Scholar]
- Molnár M., Sütö T., Tóth T., Hertelendy F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am J Obstet Gynecol. 1994 May;170(5 Pt 1):1458–1466. doi: 10.1016/s0002-9378(94)70179-2. [DOI] [PubMed] [Google Scholar]
- Nadaud S., Bonnardeaux A., Lathrop M., Soubrier F. Gene structure, polymorphism and mapping of the human endothelial nitric oxide synthase gene. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1027–1033. doi: 10.1006/bbrc.1994.1146. [DOI] [PubMed] [Google Scholar]
- Nathan L., Cuevas J., Chaudhuri G. The role of nitric oxide in the altered vascular reactivity of pregnancy in the rat. Br J Pharmacol. 1995 Mar;114(5):955–960. doi: 10.1111/j.1476-5381.1995.tb13297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. W., Allington M. J., Bolton F. G., Stirrat G. M. Plasma-beta-thromboglobulin in pre-eclampsia. Lancet. 1977 Jul 30;2(8031):248–248. doi: 10.1016/s0140-6736(77)92863-x. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Redman C. W. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993 Jun 5;341(8858):1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Parkes M., Louis E., Hashimoto L., Kato N., Welsh K., Terwilliger J. D., Lathrop G. M., Bell J. I., Jewell D. P. Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat Genet. 1996 Oct;14(2):199–202. doi: 10.1038/ng1096-199. [DOI] [PubMed] [Google Scholar]
- Sibai B. M., McCubbin J. H., Anderson G. D., Lipshitz J., Dilts P. V., Jr Eclampsia. I. Observations from 67 recent cases. Obstet Gynecol. 1981 Nov;58(5):609–613. [PubMed] [Google Scholar]
- Spielman R. S., McGinnis R. E., Ewens W. J. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet. 1993 Mar;52(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- Takimoto E., Ishida J., Sugiyama F., Horiguchi H., Murakami K., Fukamizu A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996 Nov 8;274(5289):995–998. doi: 10.1126/science.274.5289.995. [DOI] [PubMed] [Google Scholar]
- Terwilliger J. D. A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. Am J Hum Genet. 1995 Mar;56(3):777–787. [PMC free article] [PubMed] [Google Scholar]
- Ward K., Hata A., Jeunemaitre X., Helin C., Nelson L., Namikawa C., Farrington P. F., Ogasawara M., Suzumori K., Tomoda S. A molecular variant of angiotensinogen associated with preeclampsia. Nat Genet. 1993 May;4(1):59–61. doi: 10.1038/ng0593-59. [DOI] [PubMed] [Google Scholar]
- Weeks D. E., Lange K. A multilocus extension of the affected-pedigree-member method of linkage analysis. Am J Hum Genet. 1992 Apr;50(4):859–868. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wilton A. N., Cooper D. W., Brennecke S. P., Bishop S. M., Marshall P. Absence of close linkage between maternal genes for susceptibility to pre-eclampsia/eclampsia and HLA DR beta. Lancet. 1990 Sep 15;336(8716):653–657. doi: 10.1016/0140-6736(90)92149-c. [DOI] [PubMed] [Google Scholar]
- Yallampalli C., Garfield R. E. Inhibition of nitric oxide synthesis in rats during pregnancy produces signs similar to those of preeclampsia. Am J Obstet Gynecol. 1993 Nov;169(5):1316–1320. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]


