Abstract
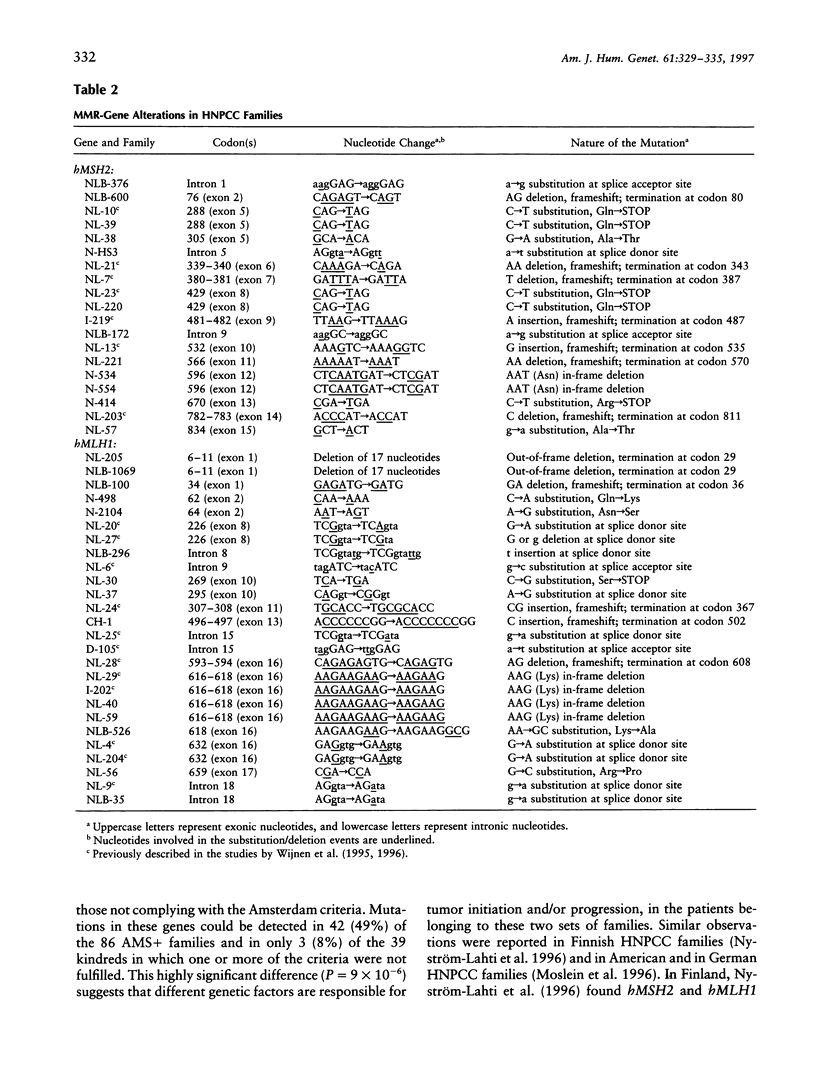
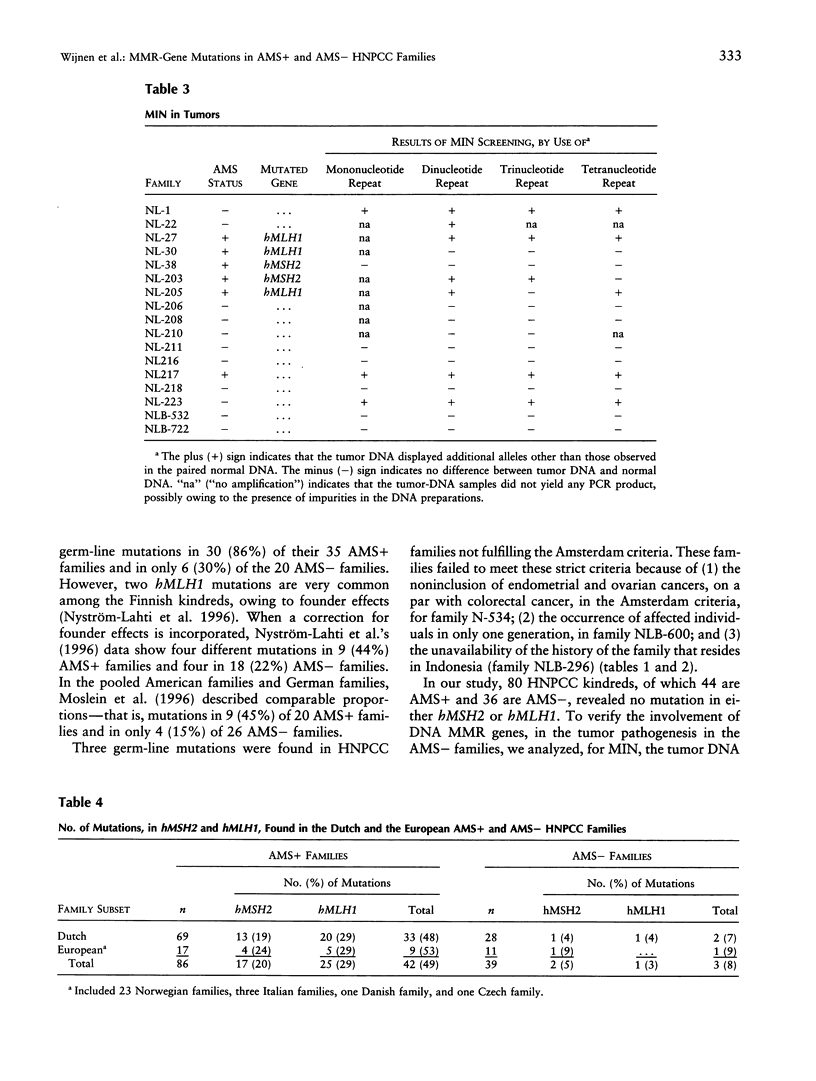
Hereditary nonpolyposis colorectal cancer (HNPCC) is a common autosomal dominant cancer-susceptibility condition characterized by early onset colorectal cancer. Germ-line mutations in one of four DNA mismatch repair (MMR) genes, hMSH2, hMLH1, hPMS1, or hPMS2, are known to cause HNPCC. Although many mutations in these genes have been found in HNPCC kindreds complying with the so-called Amsterdam criteria, little is known about the involvement of these genes in families not satisfying these criteria but showing clear-cut familial clustering of colorectal cancer and other cancers. Here, we applied denaturing gradient-gel electrophoresis to screen for hMSH2 and hMLH1 mutations in two sets of HNPCC families, one set comprising families strictly complying with the Amsterdam criteria and another set in which at least one of the criteria was not satisfied. Interestingly, hMSH2 and hMLH1 mutations were found in 49% of the kindreds fully complying with the Amsterdam criteria, whereas a disease-causing mutation could be identified in only 8% of the families in which the criteria were not satisfied fully. In correspondence with these findings, 4 of 6 colorectal tumors from patients belonging to kindreds meeting the criteria showed microsatellite instability, whereas only 3 of 11 tumors from the other set of families demonstrated this instability. Although the number of tumors included in the study admittedly is small, the frequencies of mutations in the MMR genes show obvious differences between the two clinical sets of families. These results also emphasize the practical importance of the Amsterdam criteria, which provide a valid clinical subdivision between families, on the basis of their chance of carrying an hMSH2 or an hMLH1 mutation, and which bear important consequences for genetic testing and counseling and for the management of colorectal cancer families.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Aaltonen L. A., Peltomäki P., Mecklin J. P., Järvinen H., Jass J. R., Green J. S., Lynch H. T., Watson P., Tallqvist G., Juhola M. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994 Apr 1;54(7):1645–1648. [PubMed] [Google Scholar]
- Burks R. T., Kessis T. D., Cho K. R., Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene. 1994 Apr;9(4):1163–1166. [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fodde R., Losekoot M. Mutation detection by denaturing gradient gel electrophoresis (DGGE). Hum Mutat. 1994;3(2):83–94. doi: 10.1002/humu.1380030202. [DOI] [PubMed] [Google Scholar]
- Fodde R., van der Luijt R., Wijnen J., Tops C., van der Klift H., van Leeuwen-Cornelisse I., Griffioen G., Vasen H., Khan P. M. Eight novel inactivating germ line mutations at the APC gene identified by denaturing gradient gel electrophoresis. Genomics. 1992 Aug;13(4):1162–1168. doi: 10.1016/0888-7543(92)90032-n. [DOI] [PubMed] [Google Scholar]
- Han H. J., Maruyama M., Baba S., Park J. G., Nakamura Y. Genomic structure of human mismatch repair gene, hMLH1, and its mutation analysis in patients with hereditary non-polyposis colorectal cancer (HNPCC) Hum Mol Genet. 1995 Feb;4(2):237–242. doi: 10.1093/hmg/4.2.237. [DOI] [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Hall N. R., Lipford J., Kane M. F., Morrison P. T., Finan P. J., Burn J., Chapman P., Earabino C., Merchant E. Structure of the human MLH1 locus and analysis of a large hereditary nonpolyposis colorectal carcinoma kindred for mlh1 mutations. Cancer Res. 1995 Jan 15;55(2):242–248. [PubMed] [Google Scholar]
- Lazar V., Grandjouan S., Bognel C., Couturier D., Rougier P., Bellet D., Bressac-de Paillerets B. Accumulation of multiple mutations in tumour suppressor genes during colorectal tumorigenesis in HNPCC patients. Hum Mol Genet. 1994 Dec;3(12):2257–2260. doi: 10.1093/hmg/3.12.2257. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Liu B., Parsons R., Papadopoulos N., Nicolaides N. C., Lynch H. T., Watson P., Jass J. R., Dunlop M., Wyllie A., Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996 Feb;2(2):169–174. doi: 10.1038/nm0296-169. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. C., Watson P., Lanspa S. J., Lynch J. F., Lynch P. M., Cavalieri R. J., Boland C. R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993 May;104(5):1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Moslein G., Tester D. J., Lindor N. M., Honchel R., Cunningham J. M., French A. J., Halling K. C., Schwab M., Goretzki P., Thibodeau S. N. Microsatellite instability and mutation analysis of hMSH2 and hMLH1 in patients with sporadic, familial and hereditary colorectal cancer. Hum Mol Genet. 1996 Sep;5(9):1245–1252. doi: 10.1093/hmg/5.9.1245. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Nyström-Lahti M., Wu Y., Moisio A. L., Hofstra R. M., Osinga J., Mecklin J. P., Järvinen H. J., Leisti J., Buys C. H., de la Chapelle A. DNA mismatch repair gene mutations in 55 kindreds with verified or putative hereditary non-polyposis colorectal cancer. Hum Mol Genet. 1996 Jun;5(6):763–769. doi: 10.1093/hmg/5.6.763. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Parsons R., Myeroff L. L., Liu B., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Microsatellite instability and mutations of the transforming growth factor beta type II receptor gene in colorectal cancer. Cancer Res. 1995 Dec 1;55(23):5548–5550. [PubMed] [Google Scholar]
- Risinger J. I., Berchuck A., Kohler M. F., Watson P., Lynch H. T., Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993 Nov 1;53(21):5100–5103. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Mecklin J. P., Khan P. M., Lynch H. T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991 May;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- Watson P., Lynch H. T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993 Feb 1;71(3):677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wijnen J., Khan P. M., Vasen H., Menko F., van der Klift H., van den Broek M., van Leeuwen-Cornelisse I., Nagengast F., Meijers-Heijboer E. J., Lindhout D. Majority of hMLH1 mutations responsible for hereditary nonpolyposis colorectal cancer cluster at the exonic region 15-16. Am J Hum Genet. 1996 Feb;58(2):300–307. [PMC free article] [PubMed] [Google Scholar]
- Wijnen J., Vasen H., Khan P. M., Menko F. H., van der Klift H., van Leeuwen C., van den Broek M., van Leeuwen-Cornelisse I., Nagengast F., Meijers-Heijboer A. Seven new mutations in hMSH2, an HNPCC gene, identified by denaturing gradient-gel electrophoresis. Am J Hum Genet. 1995 May;56(5):1060–1066. [PMC free article] [PubMed] [Google Scholar]
- Wooster R., Cleton-Jansen A. M., Collins N., Mangion J., Cornelis R. S., Cooper C. S., Gusterson B. A., Ponder B. A., von Deimling A., Wiestler O. D. Instability of short tandem repeats (microsatellites) in human cancers. Nat Genet. 1994 Feb;6(2):152–156. doi: 10.1038/ng0294-152. [DOI] [PubMed] [Google Scholar]


