Abstract
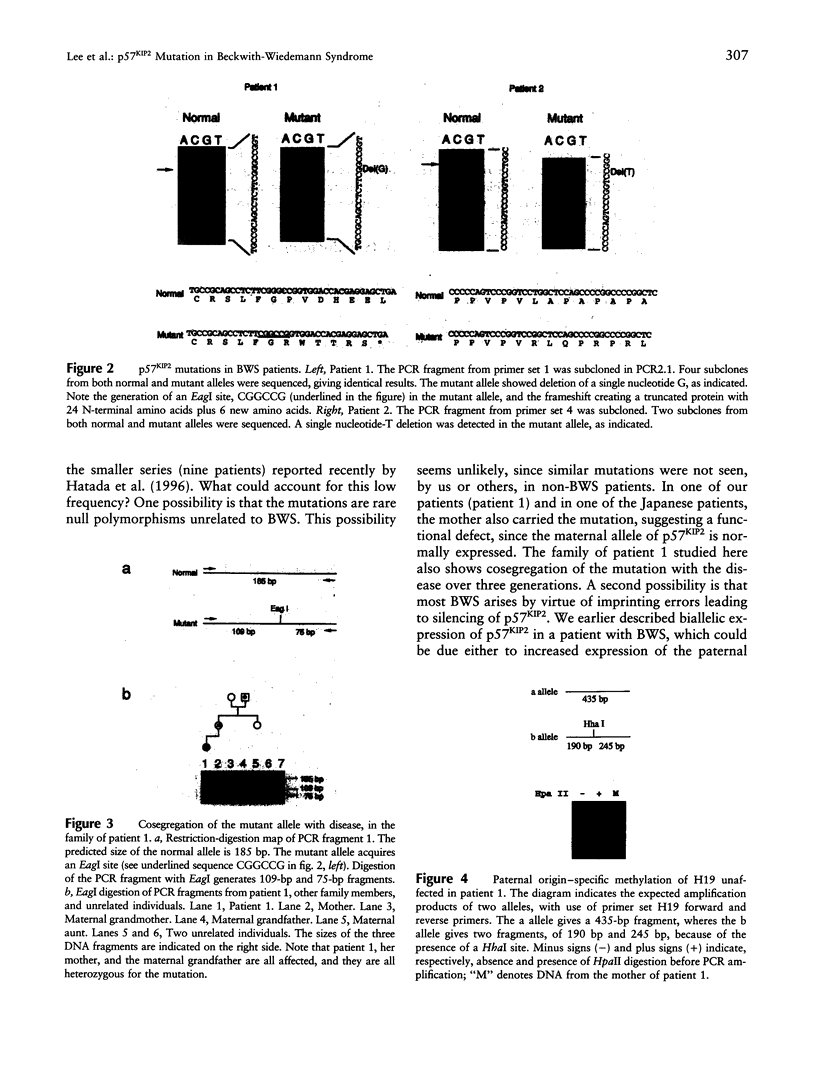
Beckwith-Wiedemann syndrome (BWS) is an autosomal dominant disorder of increased prenatal growth and predisposition to embryonal cancers such as Wilms tumor. BWS is thought to involve one or more imprinted genes, since some patients show paternal uniparental disomy, and others show balanced germ-line chromosomal rearrangements involving the maternal chromosome. We previously mapped BWS, by genetic linkage analysis, to 11p15.5, which we and others also found to contain several imprinted genes; these include the gene for insulin-like growth factor II (IGF2) and H19, which show abnormal imprint-specific expression and/or methylation in 20% of BWS patients, and p57KIP2, a cyclin-dependent kinase inhibitor, which we found showed biallelic expression in one of nine BWS patients studied. In addition, p57KIP2 was recently reported to show mutations in two of nine BWS patients. We have now analyzed the entire coding sequence and intron-exon boundaries of p57KIP2 in 40 unrelated BWS patients. Of these patients, only two (5%) showed mutations, both involving frameshifts in the second exon. In one case, the mutation was transmitted to the proband's mother, who was also affected, from the maternal grandfather, suggesting that p57KIP2 is not imprinted in at least some affected tissues at a critical stage of development and that haploinsufficiency due to mutation of either parental allele may cause at least some features of BWS. The low frequency of p57KIP2 mutations, as well as our recent discovery of disruption of the K(v)LQT1 gene in patients with chromosomal rearrangements, suggest that BWS can involve disruption of multiple independent 11p15.5 genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. W., Villar A. J., Bickmore W., Clayton-Smith J., Catchpoole D., Maher E. R., Reik W. Imprinting mutation in the Beckwith-Wiedemann syndrome leads to biallelic IGF2 expression through an H19-independent pathway. Hum Mol Genet. 1996 Dec;5(12):2027–2032. doi: 10.1093/hmg/5.12.2027. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N., Deal C., Paquette J., Goodyer C. G., Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat Genet. 1993 May;4(1):98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Hatada I., Ohashi H., Fukushima Y., Kaneko Y., Inoue M., Komoto Y., Okada A., Ohishi S., Nabetani A., Morisaki H. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996 Oct;14(2):171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- Henry I., Bonaiti-Pellié C., Chehensse V., Beldjord C., Schwartz C., Utermann G., Junien C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature. 1991 Jun 20;351(6328):665–667. doi: 10.1038/351665a0. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Sengoku K., Nakao M., Tamate K., Miyamoto T., Matsuzaka T., Sutcliffe J. S., Anan T., Takuma N., Nishiwaki K. Mouse/human sequence divergence in a region with a paternal-specific methylation imprint at the human H19 locus. Hum Mol Genet. 1996 Aug;5(8):1155–1161. doi: 10.1093/hmg/5.8.1155. [DOI] [PubMed] [Google Scholar]
- Koufos A., Grundy P., Morgan K., Aleck K. A., Hadro T., Lampkin B. C., Kalbakji A., Cavenee W. K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989 May;44(5):711–719. [PMC free article] [PubMed] [Google Scholar]
- Lee M. H., Reynisdóttir I., Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995 Mar 15;9(6):639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Lee M. P., Hu R. J., Johnson L. A., Feinberg A. P. Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements. Nat Genet. 1997 Feb;15(2):181–185. doi: 10.1038/ng0297-181. [DOI] [PubMed] [Google Scholar]
- Mannens M., Hoovers J. M., Redeker E., Verjaal M., Feinberg A. P., Little P., Boavida M., Coad N., Steenman M., Bliek J. Parental imprinting of human chromosome region 11p15.3-pter involved in the Beckwith-Wiedemann syndrome and various human neoplasia. Eur J Hum Genet. 1994;2(1):3–23. doi: 10.1159/000472337. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Edwards M. C., Bai C., Parker S., Zhang P., Baldini A., Harper J. W., Elledge S. J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995 Mar 15;9(6):650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Thompson J. S., Edwards M. C., Bartletta J. M., Grundy P., Kalikin L. M., Harper J. W., Elledge S. J., Feinberg A. P. Imprinting of the gene encoding a human cyclin-dependent kinase inhibitor, p57KIP2, on chromosome 11p15. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):3026–3030. doi: 10.1073/pnas.93.7.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton T., Crenshaw T., Hao Y., Moosikasuwan J., Lin N., Dembitzer F., Hensle T., Weiss L., McMorrow L., Loew T. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994 Jul;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- Ogawa O., Becroft D. M., Morison I. M., Eccles M. R., Skeen J. E., Mauger D. C., Reeve A. E. Constitutional relaxation of insulin-like growth factor II gene imprinting associated with Wilms' tumour and gigantism. Nat Genet. 1993 Dec;5(4):408–412. doi: 10.1038/ng1293-408. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Nyström A., Pfeifer-Ohlsson S., Töhönen V., Hedborg F., Schofield P., Flam F., Ekström T. J. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith-Wiedemann syndrome. Nat Genet. 1993 May;4(1):94–97. doi: 10.1038/ng0593-94. [DOI] [PubMed] [Google Scholar]
- Okano Y., Osasa Y., Yamamoto H., Hase Y., Tsuruhara T., Fujita H. An infant with Beckwith-Wiedemann syndrome and chromosomal duplication 11p13----pter.: correlation of symptoms between 11p trisomy and Beckwith-Wiedemann syndrome. Jinrui Idengaku Zasshi. 1986 Dec;31(4):365–372. doi: 10.1007/BF01907937. [DOI] [PubMed] [Google Scholar]
- Ping A. J., Reeve A. E., Law D. J., Young M. R., Boehnke M., Feinberg A. P. Genetic linkage of Beckwith-Wiedemann syndrome to 11p15. Am J Hum Genet. 1989 May;44(5):720–723. [PMC free article] [PubMed] [Google Scholar]
- Rainier S., Johnson L. A., Dobry C. J., Ping A. J., Grundy P. E., Feinberg A. P. Relaxation of imprinted genes in human cancer. Nature. 1993 Apr 22;362(6422):747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- Reik W., Brown K. W., Schneid H., Le Bouc Y., Bickmore W., Maher E. R. Imprinting mutations in the Beckwith-Wiedemann syndrome suggested by altered imprinting pattern in the IGF2-H19 domain. Hum Mol Genet. 1995 Dec;4(12):2379–2385. doi: 10.1093/hmg/4.12.2379. [DOI] [PubMed] [Google Scholar]
- Steenman M. J., Rainier S., Dobry C. J., Grundy P., Horon I. L., Feinberg A. P. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet. 1994 Jul;7(3):433–439. doi: 10.1038/ng0794-433. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Reese K. J., DeBaun M. R., Perlman E. J., Feinberg A. P. Reduced expression of the cyclin-dependent kinase inhibitor gene p57KIP2 in Wilms' tumor. Cancer Res. 1996 Dec 15;56(24):5723–5727. [PubMed] [Google Scholar]
- Tokino T., Urano T., Furuhata T., Matsushima M., Miyatsu T., Sasaki S., Nakamura Y. Characterization of the human p57KIP2 gene: alternative splicing, insertion/deletion polymorphisms in VNTR sequences in the coding region, and mutational analysis. Hum Genet. 1996 May;97(5):625–631. doi: 10.1007/BF02281873. [DOI] [PubMed] [Google Scholar]
- Turleau C., de Grouchy J., Chavin-Colin F., Martelli H., Voyer M., Charlas R. Trisomy 11p15 and Beckwith-Wiedemann syndrome. A report of two cases. Hum Genet. 1984;67(2):219–221. doi: 10.1007/BF00273006. [DOI] [PubMed] [Google Scholar]
- Waziri M., Patil S. R., Hanson J. W., Bartley J. A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J Pediatr. 1983 Jun;102(6):873–876. doi: 10.1016/s0022-3476(83)80014-6. [DOI] [PubMed] [Google Scholar]
- Weksberg R., Shen D. R., Fei Y. L., Song Q. L., Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993 Oct;5(2):143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]