Abstract
The direct intratumoral (i.t.) injection of anticancer agents has been evaluated extensively in the past few decades. Thus far, however, it has failed to become established as an alternative route of administration in routine clinical practice. In the present report, the impact of i.t. injection on the biodistribution and the therapeutic potential of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-based drug delivery systems was investigated. It was found that, compared to intravenous injection, both the tumor concentrations and the tumor-to-organ ratios of carriers improved substantially. In addition, compared to intravenously and intratumorally applied free doxorubicin and to intravenously applied poly(HPMA)-glycylphenylalanylleucylglycine-doxorubicin, intratumorally injected poly(HPMA)-glycylphenylalanylleucylglycine-doxorubicin presented a significantly increased antitumor efficacy, as well as an improved therapeutic index. Based on these findings, we propose intratumorally injected carrier-based chemotherapy as an interesting alternative to routinely used chemotherapy regimens and routes of administration.
Keywords: Intratumoral injection, drug delivery systems, polymer therapeutics, HPMA, chemotherapy
Introduction
Even though the direct intratumoral (i.t.) injection of anticancer agents has been evaluated extensively in the past few decades [1–5], it has not been established as an alternative route of administration in routine clinical practice. This is generally considered to be due to the invasive nature of i.t. injection, the relatively rapid clearance of topically applied drugs from tumors, and the development of dose-limiting toxicities in tissues surrounding the site of application. In addition, those types of tumors that would, in principle, be readily accessible for i.t. injection are generally being treated with more standardized (and more effective) locoregional treatment modalities, such as surgery and radiotherapy.
Alongside advances in establishing novel antitumor therapeutics, a large number of drug delivery systems have been developed over the years, both for parenteral and for topical administration [6–10]. Thus far, however, even though several highly innovative delivery systems have been designed specifically for locoregional application, only very few have managed to progress into clinical trials. Taking this observation into account, we set out to evaluate the impact of i.t. injection on the biodistribution and therapeutic potential of N-(2-hydroxypropyl)-methacrylamide (HPMA) copolymer-based drug delivery systems. Copolymers of HPMA are prototypic and well-characterized polymeric drug carriers that have been broadly implemented in the delivery of anticancer therapeutics and that have been tested in several phase I and phase II clinical trials [11–15].
In the first set of experiments, the circulation kinetics, organ distribution, and tumor localization of intravenously and intratumorally applied HPMA copolymers were investigated in rats bearing subcutaneously transplanted Dunning AT1 tumors. Subsequently, the impact of i.t. injection on the biodistribution of poly(HPMA)-glycylphenylalanylleucylglycine (GFLG)-doxorubicin was assessed to evaluate whether the effects observed for chemically unmodified HPMA copolymers also hold for a clinically relevant HPMA copolymer carrying a chemotherapeutic drug. Finally, the antitumor efficacy, toxicity, and therapeutic index of intravenously and intratumorally applied poly(HPMA)-GFLG-doxorubicin were analyzed, and they were compared to those of intravenously and intratumorally applied free doxorubicin.
It was found that i.t. injection substantially improved both the tumor concentrations and the tumor-to-organ ratios of copolymers. In addition, compared to intravenously and intratumorally applied free doxorubicin and to intravenously applied poly(HPMA)-GFLG-doxorubicin, intratumorally administered poly(HPMA)-GFLG-doxorubicin improved both the efficacy and the toxicity of a single dose of chemotherapy. Based on these findings, we propose that—when advanced solid malignancies are easily accessible (e.g., intraoperatively)—intratumorally administered carrier-based chemotherapy be considered as an alternative to routinely used chemotherapy regimens and routes of administration.
Materials and Methods
Chemicals
Methacryloyl chloride, methacrylic acid, 1-aminopropan-2-ol, tyrosine amide, glycylglycine, glycylphenylalanine, leucylglycine, 4-nitrophenol, dimethylsulfoxide (DMSO), and doxorubicin hydrochloride were obtained from Fluka (Prague, Czech Republic) and were of appropriate analytic grade.
Synthesis and Characterization of Copolymers
The HPMA copolymers used in this study were synthesized as described previously [16]. Briefly, poly(HPMA-co-MA-TyrNH2) was prepared by the solution radical copolymerization of the monomers HPMA and MA-TyrNH2 in methanol. The weight-average molecular weight (Mw), number-average molecular weight (Mn), and polydispersity (Mw/Mn) of copolymers after their fractionation (on Superose 4B/6B columns, Amersham Biosciences, Prague, Czech Republic) were determined by size exclusion chromatography on an Äkta Explorer (Amersham Biosciences) equipped with UV-VIS, a differential refractometer (Shodex R-72, Kawasaki, Japan), and a multiangle light scattering detector (DAWN DSP-F; Wyatt Technology Corp., Santa Barbara, CA). The average molecular weights of the two parental HPMA copolymers were 30.5 and 64.5 kDa, respectively; their polydispersities were 1.3 and 1.2, respectively; and the relative amounts of tyrosine amide, included to allow for radiolabeling, were 0.8 and 0.3 mol%, respectively.
The precursor for poly(HPMA)-GFLG-doxorubicin [i.e., poly(HPMA)-co-MA-TyrNH2-co-MA-Gly-dl-PheLeuGly-ONp] was prepared by the precipitation radical terpolymerization of HPMA, MA-TyrNH2, and MA-GFLG-ONp in acetone. After purification, doxorubicin was conjugated to the precursor in DMSO, in the presence of Et3N. The reaction mixture was stirred for 4 hours, 1-aminopropan-2-ol was added, and the mixture was precipitated into a mixture of acetone/diethylether (3:1). The resulting doxorubicin-containing conjugate was then filtered off, dried in vacuum, purified on a Sephadex LH-20 column (Sigma-Aldrich, Prague, Czech Republic) (to remove free doxorubicin), and purified on a Sephadex LH-60 column (Sigma-Aldrich) (to obtain a narrow distribution of molecular weights). The molecular weight of the conjugate was 27.9 kDa, its polydispersity was 1.5, the amount of MA-TyrNH2 was 1.3 mol%, and the amount of doxorubicin was 6.5 wt.%.
Radiolabeling
Iodine-131 (131I) was obtained from Amersham (Freiburg, Germany). The tyrosine amide groups incorporated into copolymers were radiolabeled using the mild oxidizing agent 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (i.e., Iodogen [17]). On 10 minutes of incubation, the mixture of 131I, Iodogen, and copolymer was applied to a Biogel-P6 column (Bio-Rad Laboratories, München, Germany) and eluted with 30 ml of phosphate-buffered saline. The eluate was recovered in 1-ml fractions, and the radioactivity of each of these fractions was determined by a scintillation counter. The radiolabeled copolymer was retrieved in the fifth to the seventh milliliter of the eluate, whereas free (i.e. inbound) 131I was eluted in the fourteenth to the eighteenth milliliter. As this methodology allowed us to concentrate the copolymer-associated fraction, no additional purification was required. The efficacy of radiolabeling was quantified by dividing the amount of radioactivity collected in the fifth to the seventh milliliter of the eluate by the total amount of radioactivity retrieved (i.e., by the sum of the activities detected in all thirty 1-ml fractions). The labeling efficacies for 31-kDa poly(HPMA), 65-kDa poly(HPMA), and 28-kDa poly(HPMA)-GFLG-doxorubicin were 95.2%, 96.2%, and 86.3%, respectively.
Animal Model
All experiments involving animals were approved by an external committee for animal welfare and were performed according to the guidelines for laboratory animals established by the German Government. Experiments were performed on 6- to 12-month-old male Copenhagen rats (Charles River WIGA, Sulzfeld, Germany) using the syngeneic Dunning R3327-AT1 prostate carcinoma model [18]. Throughout the experimental procedure, the animals were anesthetized with Ethrane (DeltaSelect, Pfullingen, Germany). Fresh pieces of AT1 tumor tissue (≈ 10 mm3) were prepared from an AT1 donor tumor and were transplanted subcutaneously into the right hindlimbs of the rats. Tumors were grown for 12 to 18 days until they had reached an average diameter of 12 mm.
Biodistribution
To analyze the biodistribution of the copolymers on intravenous (i.v.) injection, 500 µl of a saline solution containing 0.1 mmol of HPMA copolymer (based on copolymer concentration and corresponding to a radioactivity of 150–300 µCi) was injected intravenously into the lateral tail vein of the animals. The biodistribution of the copolymers on i.t. injection was evaluated by administering a substantially smaller volume (50–100 µl) containing the same amount of copolymer (i.e., 0.1 mmol; 150–300 µCi) directly into the center of the tumors. Immediately after i.t. injection, the application site was covered and washed twice with absorbing paper to retrieve the radiolabeled copolymer leaking out of the tumor. At 0.1, 0.25, 1, 4, and 24 hours postinjection (p.i.), the concentrations of the copolymers in systemic circulation were determined by withdrawing 50 µl of blood from the tail vein of the rats and by assuming that the complete blood pool equals 6% of their body weight. At 0.5, 4, and 24 hours p.i., the biodistribution of the copolymers was monitored two-dimensionally using a Searle-Siemens (Erlangen, Germany) scintillation camera. At 24 hours p.i., the animals were sacrificed, and their tumors and organs were harvested for quantification. The residual amounts of radioactivity were determined using a gamma counter, corrected for radioactive decay, and expressed as percentage of injected dose per gram of tissue (% ID/g).
In Vitro Efficacy
The cytotoxicity of free and HPMA copolymer-bound doxorubicin was determined by seeding 200 Dunning AT1 cells into six-well plates. Four hours later, the cells were treated with 0.001 to 10 µmol of free doxorubicin, 0.001 to 1000 µmol of poly(HPMA)-GFLG-doxorubicin, and 0.001 to 1000 µmol of a drug-free control copolymer. After 8 to 10 days, the cells were fixed and stained with crystal violet, and the number of surviving colonies was counted.
Antitumor Efficacy
Rats bearing 10- to 15-mm AT1 tumors were randomly assigned to various treatment groups. Free and HPMA copolymer-bound doxorubicin were administered by a single i.v. or i.t injection at a (doxorubicin-equivalent) dose of 5 mg/kg. Tumor volumes were calculated using the formula: V = [a(bb)]/2, where a is the largest diameter and b is the smallest diameter, and they were expressed relative to the tumor volume determined on the first day of therapy. The toxicity of the four regimens was assessed by measuring the relative body weight (loss) of the animals.
Statistical Analysis
All values are expressed as average ± SD. In the experiments addressing the kinetics, biodistribution, and tumor localization of the copolymers, the standard Student's t test was used. In the experiments evaluating the efficacy and the toxicity of the various treatment regimens, the Mann-Whitney U test was used. In both cases, P < .05 was considered statistically significant.
Results
Effect of i.t. Injection on the Kinetics of HPMA Copolymers
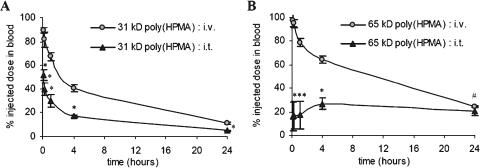
First, the impact of i.t. injection on the circulation kinetics of two chemically unmodified HPMA copolymers (i.e., without spacer and drug) was investigated. Hereto, 31-kDa and 65-kDa poly(HPMA) were radiolabeled and administered to the rats either as an i.v. bolus injection or directly into the center of the tumors. Figure 1A shows that, up to 24 hours p.i., the blood concentrations of intratumorally applied 31-kDa poly(HPMA) were significantly lower than those of intravenously applied 31-kDa poly(HPMA). At 1 and 24 hours p.i., for instance, 30.1 ± 5.2% and 4.9 ± 0.7% ID were found in blood on i.t. administration, compared to 67.4 ± 3.3% ID (P < .0001) and 11.2 ± 0.7% ID (P < .0001) on i.v. administration.
Figure 1.
Effect of i.t. injection on the circulation kinetics of HPMA copolymers. The blood concentrations of 31-kDa poly(HPMA) (A) and 65-kDa poly(HPMA) (B) after i.v. and i.t. injection are plotted against time. Values represent the average ± SD of four to six animals per experimental group. *P < .0001 vs i.v. injection. #P < .05 vs i.v. injection (Student's t test).
Figure 1B shows that, also for 65-kDa poly(HPMA), the levels in the systemic circulation were significantly lower on i.t. injection. At 1 and 24 hours p.i., 17.2 ± 11.6% and 20.5 ± 3.7% ID were found on i.t. administration vs 78.8 ± 3.4% ID (P < .0001) and 24.0 ± 0.8% ID (P < .05) on i.v. administration, respectively. Compared to 31-kDa poly(HPMA), a different pharmacokinetic pattern was observed for 65-kDa poly(HPMA). For the smaller copolymer, the concentrations in blood were found to be relatively high immediately on i.t. injection (∼50% ID at 5 minutes p.i.) and gradually decreased over time. For the larger copolymer, however, the initial levels were relatively low (∼10% ID at 5 minutes p.i.) and they tended to remain constant over time. This indicates that larger HPMA copolymers are retained in tumor more effectively than smaller HPMA copolymers.
Effect of i.t. Injection on the Biodistribution of HPMA Copolymers
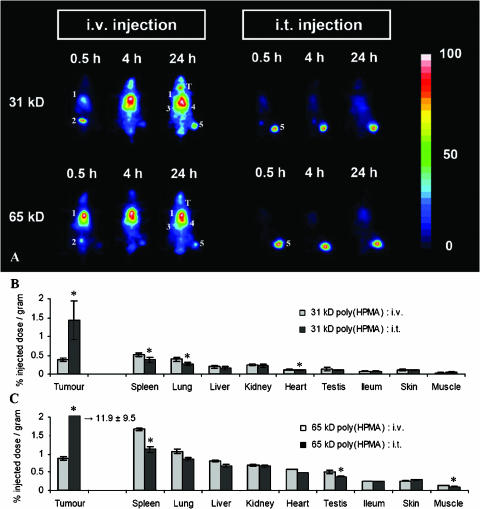
Next, the tumor localization and organ distribution of intravenously and intratumorally applied HPMA copolymers were compared. As shown in the scintigrams in Figure 2A, at 4 and 24 hours after i.v. injection, alongside a substantial accumulation in AT1-sc tumors, significant amounts of the copolymers were also found in the heart (i.e., circulation), spleen, and liver. On i.t. application, however, only localization to the tumors could be observed over the first 24 hours after administration (Figure 2A).
Figure 2.
Effect of i.t. injection on the biodistribution of HPMA copolymers. (A) Scintigraphic analysis of the effect of i.t. injection on the biodistribution of 31-kDa and 65-kDa poly(HPMA) in rats bearing subcutaneously transplanted AT1 tumors. In the images obtained 0.5 hour after i.v. administration, the accumulation of the radiolabeled copolymers was most prominent in the heart (i.e., circulation) (1) and bladder (2). In the images obtained at 4 and 24 hours, the highest amounts of the copolymers were found in the heart/lungs (1), spleen (3), liver (4), and tumor (5). In addition, at the two latter time points, released radioactive iodine was found to accumulate in the thyroid (T). On i.t. injection, only localization to the tumor (5) could be observed over the first 24 hours after administration. (B and C) Quantification of the effect of i.t. injection on the tumor and organ concentrations of 31-kDa poly(HPMA) (B) and 65 kDa-poly(HPMA) (C) at 24 hours p.i. Values represent the average ± SD of four to six animals per experimental group. *P < .05 vs i.v. injection (Student's t test).
In addition, the scintigrams in Figure 2A point toward an accumulation of radioactivity in the thyroid. This, however, is due to the release of 131I from copolymers; under physiological conditions, a small amount of radiolabel is liberated from incorporated tyrosine amide groups (approximately 2% per 24 hours). Most of this released 131I is eliminated rapidly by renal filtration; a significant portion, however, is always taken up by thyroid cells, as these cells specifically express the sodium-iodine symporter.
At 24 hours p.i., the tumors and organs were then harvested, and the concentrations of the copolymers were quantified. Figure 2, B and C, shows that, on i.v. injection, the highest amounts of the copolymers were always detected in the spleen, followed by the lungs and tumor. For 31-kDa poly(HPMA), the levels localizing to the tumor were 0.38 ± 0.03% ID/g for i.v. injection and 1.42 ± 0.52% ID/g for i.t. injection (P = .0023). In the spleen, lungs, and liver, the concentrations of intravenously applied 31-kDa poly(HPMA) were 0.52 ± 0.04%, 0.39 ± 0.06%, and 0.19 ± 0.03% ID/g, respectively, compared to 0.38 ± 0.06%, 0.26 ± 0.04%, and 0.15 ± 0.04% ID/g, respectively, for intratumorally applied 31-kDa poly(HPMA). These findings indicate that, in addition to increasing the tumor concentrations of this copolymer, i.t. injection also decreases its localization to healthy tissues. Overall, however, the differences were less obvious than predicted by the scintigrams, and they were only found to be significant for the spleen (P = .0043), lungs (P = .0039), and heart (P = .0026).
For 65-kDa poly(HPMA), an identical biodistributional pattern was observed. As shown in Figure 2C, 24 hours after i.v. injection, the highest concentrations of the copolymer were found in the spleen (1.67 ± 0.06% ID/g), lungs (1.06 ± 0.15% ID/g), tumor (0.87 ± 0.06% ID/g), and liver (0.80 ± 0.05% ID/g). On i.t. injection, its levels in these tissues were 1.12 ± 0.37% ID/g (P = .0059), 0.86 ± 0.35% ID/g (P = .2314), 11.9 ± 9.5% ID/g (P = .0195), and 0.66 ± 0.19% ID/g (P = .0971), respectively. Thus, as for 31-kDa poly(HPMA), i.t. injection not only increased the tumor accumulation of 65-kDa poly(HPMA) but also attenuated its localization to several healthy tissues.
To more directly assess the effects of i.t. injection on the biodistribution of the copolymers, tumor-to-organ ratios were calculated. Hereto, the tumor concentrations of 31-kDa and 65-kDa poly(HPMA) at 24 hours p.i. were divided by the respective organ concentrations at 24 hours p.i. As shown in Table 1, the tumor-to-organ ratios of intratumorally applied 31-kDa poly(HPMA) were, on average, four-fold higher than those of intravenously applied 31-kDa poly(HPMA). For 65-kDa poly(HPMA), i.t. injection improved the tumor-to-organ ratios by a factor 15 to 20. These findings indicate that the (positive) impact of i.t. injection correlates with the molecular weight of copolymers.
Table 1.
Summary of the Effects of i.t. Injection on the Biodistribution and Therapeutic Potential of HPMA Copolymer-Based Drug Delivery Systems: Evaluation of the Tumor-to-Organ Ratios of Intravenously and Intratumorally Applied HPMA Copolymers.
| 31-kDa poly(HPMA) | 65-kDa poly(HPMA) | 28-kDa poly(HPMA)-GFLG-Doxorubicin | ||||
| i.v. | i.t. | i.v. | i.t. | i.v. | i.t. | |
| Tumor | 1 | 1 | 1 | 1 | 1 | 1 |
| Spleen | 0.7 | 3.8 | 0.5 | 10.6 | 0.2 | 1.9 |
| Lungs | 1.0 | 5.5 | 0.8 | 13.8 | 1.2 | 8.5 |
| Liver | 2.0 | 9.4 | 1.1 | 18.0 | 1.3 | 8.1 |
| Kidneys | 1.6 | 6.3 | 1.3 | 18.1 | 0.1 | 0.5 |
| Heart | 3.2 | 14.1 | 1.5 | 24.6 | 3.2 | 15.6 |
| Testes | 2.8 | 13.7 | 1.7 | 32.0 | 2.8 | 14.8 |
| Skin | 5.0 | 19.7 | 3.5 | 49.0 | 1.6 | 9.4 |
| Ileum | 3.5 | 13.6 | 3.3 | 40.3 | 2.5 | 19.3 |
| Muscle | 12.0 | 36.6 | 6.2 | 120.7 | 7.6 | 45.7 |
Tumor-to-organ ratios were calculated 24 hours after i.v. and i.t. injection. Hereto, the tumor concentrations of 31-kDa poly(HPMA), 65-kDa poly(HPMA), and 28-kDa poly(HPMA)-GFLG-doxorubicin at 24 hours p.i. were divided by the respective organ concentrations at 24 hours p.i. A tumor-to-organ ratio of >1 indicates a preferred localization to tumor tissues; a ratio of <1 indicates a more selective localization to the corresponding healthy tissues. The tumor-to-organ ratios allow for a more direct evaluation of the impact of i.t. injection on the biodistribution of the copolymers.
Effect of i.t. Injection on the Biodistribution of Poly(HPMA)-GFLG-Doxorubicin
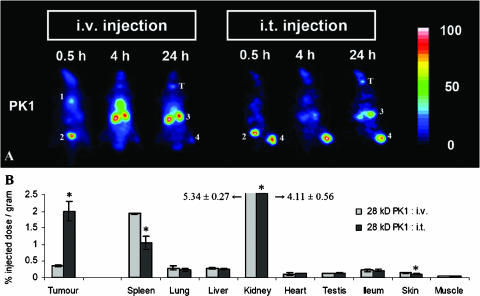
To evaluate whether the effects observed for the two chemically unmodified copolymers also hold for a clinically relevant HPMA copolymer carrying a chemotherapeutic drug, we also analyzed the impact of i.t. injection on the bio-distribution of poly(HPMA)-GFLG-doxorubicin. As shown in the scintigrams in Figure 3A, the biodistribution of intravenously applied poly(HPMA)-GFLG-doxorubicin seems to be very different from that observed for the two parental copolymers (Figure 2A). In a previous study, however, we have shown that, as a result of the incorporation of drug and/or spacer moieties, the kidney concentrations of the copolymers are always induced significantly (∼ 5- to 10-fold), whereas their relative levels in the majority of other tissues are affected only moderately [16]. The scintigrams in Figure 3A furthermore exemplify that, as for the two parental HPMA copolymers, i.t. injection substantially improved the tumor localization of poly(HPMA)-GFLG-doxorubicin. Quantification at 24 hours p.i. confirmed this notion, showing that 2.00 ± 0.28% ID/g was found for i.t. administration, compared to 0.36 ± 0.02% ID/g (P = .0006) for i.v. administration (Figure 3B). Figure 3B also shows that i.t. injection reduced the amount of poly(HPMA)-GFLG-doxorubicin accumulating in the spleen (P = .0018), kidneys (P = .0261), and skin (P = .0091). As a result, the tumor-to-organ ratios of intratumorally applied poly(HPMA)-GFLG-doxorubicin were found to be substantially higher than those of intravenously applied poly(HPMA)-GFLG-doxorubicin; on average, they improved by more than 500% (Table 1).
Figure 3.
Effect of i.t. injection on the biodistribution of poly(HPMA)-GFLG-doxorubicin. (A) Scintigraphic analysis of the effect of i.t. injection on the biodistribution of poly(HPMA)-GFLG-doxorubicin (PK1) in rats bearing subcutaneously transplanted AT1 tumors. In the images obtained 0.5 hour after i.v. injection, the accumulation of the radiolabeled conjugate was most prominent in the heart (i.e., circulation) (1) and bladder (2). At 4 and 24 hours, most of the conjugate was found in the kidneys (3) and tumor (4). Released radioactive iodine was again found to accumulate in the thyroid (T). On i.t. injection, the highest amounts of poly(HPMA)-GFLG-doxorubicin were found in the kidneys (3) and tumor (4). (B) Quantification of the effect of i.t. injection on the tumor and organ concentrations of poly(HPMA)-GFLG-doxorubicin (PK1) at 24 hours p.i. Values represent the average ± SD of three to four animals per experimental group. *P < .05 vs i.v. injection (Student's t test).
In Vitro Efficacy of Free and HPMA Copolymer-Bound Doxorubicin
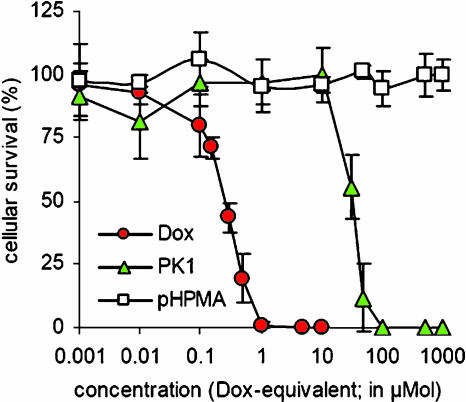
Subsequently, the cytotoxicity of poly(HPMA)-GFLG-doxorubicin was compared to that of free doxorubicin. As shown in Figure 4, free doxorubicin was found to be significantly more effective than poly(HPMA)-GFLG-doxorubicin in inhibiting the clonogenic survival of AT1 cells. In line with the literature [19,20], the IC50 value of the free drug (∼ 0.3 µmol) was approximately 100-fold lower than that of the copolymerbound drug (∼ 30 µmol). For a drug-free control copolymer, no cytotoxic effects were observed.
Figure 4.
In vitro efficacy of free and HPMA copolymer-bound doxorubicin. The cytotoxicity of free doxorubicin, poly(HPMA)-GFLG-doxorubicin, and control copolymer (lacking doxorubicin) was assessed by investigating the ability of agents to inhibit the colony formation of AT1 rat prostate carcinoma cells. Values represent the average ± SD of three independent experiments.
Effect of i.t. Injection on the Efficacy and Toxicity of Free and HPMA Copolymer-Bound Doxorubicin
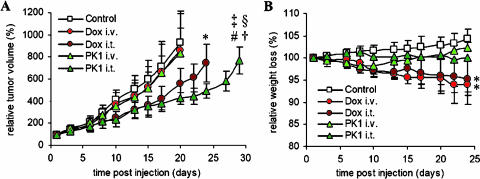
Finally, the therapeutic efficacy of intravenously and intratumorally applied poly(HPMA)-GFLG-doxorubicin (PK1) was compared to that of intravenously and intratumorally applied free doxorubicin. As shown in Figure 5A, neither a single i.v. injection of free doxorubicin nor a single i.v. injection of PK1 was able to inhibit the growth of aggressively growing and relatively chemoresistant Dunning AT1 tumors. When free doxorubicin was applied directly into the tumors, it was only found to be significantly more effective than control (P = .02). Intratumorally applied PK1, however, was not only found to be more effective than control (P = .004) but also found to be more effective than intravenously applied free doxorubicin (P = .005), intratumorally applied free doxorubicin (P = .03), and intravenously applied PK1 (P = .008).
Figure 5.
Effect of i.t. injection on the efficacy and toxicity of free and HPMA copolymer-bound doxorubicin. (A) Growth inhibition of subcutaneous AT1 tumors induced by a single i.v. injection of saline (Control; n = 12), a single i.v. injection of 5 mg/kg free doxorubicin (Dox i.v.; n = 9), a single i.t. injection of 5 mg/kg doxorubicin (Dox i.t.; n = 4), a single i.v. injection of 5 mg/kg (doxorubicin-equivalent) poly(HPMA)-GFLG-doxorubicin (PK1 i.v.; n = 7), and a single i.t. injection of 5 mg/kg (doxorubicin-equivalent) poly(HPMA)-GFLG-doxorubicin (PK1 i.t.; n = 4). *P < .05 vs control. #P < .005 vs control. †P < .01 vs Dox i.v. ‡P < .01 vs PK1 i.t. §P < .05 vs DOX i.t. (Mann-Whitney U test). (B) Weight loss induced by the four chemotherapy regimens mentioned above. *P < .05 vs control and PK1 i.v. (Mann-Whitney U test).
In addition to evaluating the effect of i.t. injection on the antitumor efficacy of free and HPMA copolymer-bound doxorubicin, we also investigated its impact on the toxicity of the two chemotherapeutic agents. Hereto, the body weight (loss) of the animals was monitored throughout the course of the experiment. As shown in Figure 5B, intravenously and intratumorally applied PK1 turned out to be better tolerated than intravenously and intratumorally applied free doxorubicin; although the toxicity resulting from the two regimens involving PK1 was comparable to that of control, both regimens involving free doxorubicin were found to be significantly more toxic than control (P = .01).
To more directly compare the overall therapeutic potential of intravenously and intratumorally applied PK1 to that of intravenously and intratumorally applied free doxorubicin, therapeutic indices were attributed to the four chemotherapy regimens. Hereto, the relative increases in efficacy (i.e., in tumor growth inhibition; compared to control) were divided by the relative increases in toxicity (i.e., in body weight loss; compared to control). On day 10 p.i., for instance, the relative tumor volumes for control and intratumorally applied PK1 were 417% and 238%, respectively (Figure 5A). The relative body weights for these two regimens at this time point were 102% and 100%, respectively (Figure 5B). The resulting therapeutic index for intratumorally applied PK1 on day 10 is thus 1.72 [i.e., (417/238) / (102/100)]. As shown in Table 2, when simultaneously addressing the efficacy and the toxicity of a single dose of chemotherapy, intratumorally applied PK1 turned out to be the most optimal regimen for treating Copenhagen rats carrying chemoresistant AT1 tumors; throughout follow-up, its therapeutic indices were always well above 1 (i.e., better than those of saline controls) and they were also always substantially higher than those determined for the other three chemotherapy regimens.
Table 2.
Summary of the Effects of i.t. Injection on the Biodistribution and Therapeutic Potential of HPMA Copolymer-Based Drug Delivery Systems: Analysis of the Therapeutic Index of Intravenously and Intratumorally Applied Free and HPMA Copolymer-Bound Doxorubicin.
| Time (days) | |||||||||
| 1 | 3 | 6 | 8 | 10 | 13 | 15 | 17 | 20 | |
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Dox i.v. | 1 | 1.12 | 1.09 | 1.14 | 1.06 | 1.07 | 1.07 | 1.00 | 1.00 |
| Dox i.t. | 1 | 1.00 | 1.19 | 1.30 | 1.56 | 1.50 | 1.58 | 1.59 | 1.56 |
| PK1 i.v. | 1 | 0.95 | 0.97 | 1.06 | 1.13 | 1.14 | 1.12 | 1.06 | 1.08 |
| PK1 i.t. | 1 | 1.17 | 1.24 | 1.37 | 1.72 | 1.55 | 1.67 | 1.85 | 2.13 |
Therapeutic indices were determined for each of the four chemotherapy regimens throughout the course of the experiment. To quantify therapeutic indices, relative increases in efficacy (i.e., in tumor growth inhibition; compared to saline control) were divided by relative increases in toxicity (i.e., in body weight loss; compared to saline control; see text for details). The assessment of therapeutic indices is intended to allow for a more direct and cross-sectional comparison of the overall therapeutic potential of the four chemotherapy regimens.
Discussion
Besides being the standard route of administration for most (pre)clinical gene therapy applications [21 22], i.t. injection has also been evaluated relatively extensively for improving the therapeutic index of standard anticancer agents [1–5,23–25]. The obvious rationale behind this approach is that the topical administration of chemotherapeutic drugs increases the concentrations of agents at the target site, while lowering their localization to healthy tissues. As a result, i.t. injection is generally considered to improve the antitumor efficacy of agents, while lowering the incidence and the intensity of their side effects.
In principle, the rationale behind the implementation of drug delivery systems is identical to that of i.t. injection: to improve the therapeutic index of chemotherapeutic agents by increasing their tumor concentrations and by decreasing their accumulation in healthy tissues [6–10]. It therefore seems logical that the combination of these two approaches (i.e. the i.t. injection of drug delivery systems) holds significant potential for further enhancing the efficacy of anticancer therapy. Thus far, however, this combination has been largely neglected, and only very few reports have evaluated the impact of i.t. injection on the biodistribution and therapeutic index of carrier-based chemotherapeutics. Those reports that did investigate the efficacy of the combination have convincingly confirmed its potential; carmustine-containing polymeric wafers designed specifically for (intraoperative) intracerebral administration, for instance, have been shown to substantially improve both the efficacy and the tolerability of chemotherapy, and, consequently, they have been approved by the Food and Drug Administration for the treatment of glioblastoma [26–28].
Surely, drug delivery systems designed specifically for topical administration can be expected to be more suitable for i.t. administration than are delivery systems designed for parenteral administration (e.g., HPMA copolymers). Based on the notion, however, that HPMA copolymers are clinically relevant drug carriers and that they are known to display proper biocompatibility and enhanced tumor retention [8, 11–16], we decided to use HPMA copolymers to demonstrate that, even by implementing drug delivery systems that were designed specifically for parenteral administration, the therapeutic index of locoregionally applied chemotherapy can be improved substantially. On one hand, this was performed to urge oncologists considering intraoperative or postoperative chemotherapy to use carrier-based chemotherapeutics instead of standard chemotherapeutics. On the other hand, this should also serve as a starting point and as a rationale for intensifying the evaluation of drug delivery systems designed specifically for topical administration.
In the present report, several lines of evidence indicating that the implementation of drug delivery systems is indeed a promising approach for improving the efficacy of locoregionally applied anticancer therapy are provided. First, the blood concentrations of intratumorally injected drug delivery systems were found to be significantly lower than those of intravenously injected delivery systems. This indicates that the systemic toxicity of intratumorally applied (carrier-based) chemotherapy can be expected to be lower than that of intravenously applied (carrier-based) chemotherapy. Second, in line with the experimental evidence provided by Harrington et al. [29], who showed that the i.t. administration of colloidal drug carriers substantially increases their tumor concentrations, we found that i.t. injection also substantially improves the biodistribution of polymeric drug delivery systems (Figures 2 and 3). Compared to i.v. injection, the tumor-to-organ ratios resulting from i.t. injection increased by up to 2000% (Table 1). Third, most likely as a direct result of this improved tumor localization, the antitumor efficacy of intratumorally applied poly(HPMA)-GFLG-doxorubicin was found to be significantly higher than that of intravenously applied poly(HPMA)-GFLG-doxorubicin and intravenously and intratumorally applied free doxorubicin (Figure 5A). At the same time, the toxicity of this regimen turned out to be attenuated (Figure 5B), resulting in a substantial improvement in the overall therapeutic index of the intervention (Table 2). These findings indicate that when advanced solid malignancies are easily accessible (e.g., intraoperatively), the i.t. injection of HPMA copolymer-based chemotherapeutics—and likely of all carrier-based chemotherapeutics—should be considered as an interesting alternative to routinely used chemotherapy regimens and routes of administration.
Acknowledgements
Lutz Edler is gratefully acknowledged for assistance with statistical analyses, Ditmar Greulich for in vitro analyses, and Jochen Schumacher for radiolabeling of the copolymers.
Abbreviations
- HPMA
N-(2-hydroxypropyl)methacrylamide
- i.t.
intratumoral
- i.v.
intravenous
- p.i.
postinjection
Footnotes
This work was supported by the German-Israeli Cooperation Program in Cancer Research (T.L. and P.P.), the Wieland-Stiftung (T.L.), and grant GACR 204/05/2255 (V.S. and K.U.).
The authors declare no competing financial interests.
References
- 1.Brincker H. Direct intratumoral chemotherapy. Crit Rev Oncol Hematol. 1993;15:91–98. doi: 10.1016/1040-8428(93)90049-a. [DOI] [PubMed] [Google Scholar]
- 2.Walter KA, Tamargo RJ, Olivi A, Burger PC, Brem H. Intratumoral chemotherapy. Neurosurgery. 1995;37:1128–1145. [PubMed] [Google Scholar]
- 3.Voulgaris S, Partheni M, Karamouzis M, Dimopoulos P, Papadakis N, Kalofonos HP. Intratumoral doxorubicin in patients with malignant brain gliomas. Am J Clin Oncol. 2002;1:60–64. doi: 10.1097/00000421-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg EP, Hadba AR, Almond BA, Marotta JS. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. J Pharm Pharmacol. 2002;54:159–180. doi: 10.1211/0022357021778268. [DOI] [PubMed] [Google Scholar]
- 5.Duvillard C, Romanet P, Cosmidis A, Beaudouin N, Chauffert B. Phase 2 study of intratumoral cisplatin and epinephrine treatment for locally recurrent head and neck tumors. Ann Otol Rhinol Laryngol. 2004;113:229–233. doi: 10.1177/000348940411300312. [DOI] [PubMed] [Google Scholar]
- 6.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 7.Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell. 2003;4:337–341. doi: 10.1016/s1535-6108(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 8.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 9.Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005;11:8230–8234. doi: 10.1158/1078-0432.CCR-05-1895. [DOI] [PubMed] [Google Scholar]
- 10.Braun K, Pipkorn R, Waldeck W. Development and characterization of drug delivery systems for targeting mammalian cells and tissues: a review. Curr Med Chem. 2005;12:1841–1858. doi: 10.2174/0929867054546672. [DOI] [PubMed] [Google Scholar]
- 11.Kopecek J, Kopeckova P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur J Pharm Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 12.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, Schot M, Mandjes IA, Zurlo MG, Rocchetti M, Rosing H, Koopman FJ, Beijnen JH. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs. 2001;12:315–323. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bilim V. Technology evaluation: PK1, Pfizer/Cancer Research UK. Curr Opin Mol Ther. 2003;5:326–330. [PubMed] [Google Scholar]
- 14.Rihova B, Kubackova K. Clinical implications of N-(2-hydroxypropyl)-methacrylamide copolymers. Curr Pharm Biotechnol. 2003;4:311–322. doi: 10.2174/1389201033489711. [DOI] [PubMed] [Google Scholar]
- 15.Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, De Boer RF, Pluim D, Beijnen JH, Schellens JH, Droz JP. A Phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors. Clin Cancer Res. 2004;10:3386–3395. doi: 10.1158/1078-0432.CCR-03-0315. [DOI] [PubMed] [Google Scholar]
- 16.Lammers T, Kuhnlein R, Kissel M, Subr V, Etrych T, Pola R, Pechar M, Ulbrich K, Storm G, Huber P, et al. Effect of physicochemical modification on the biodistribution and tumor accumulation of HPMA copolymers. J Control Release. 2005;110:103–118. doi: 10.1016/j.jconrel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Salacinski PR, McLean C, Sykes JE, Clement-Jones VV, Lowry PJ. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3 alpha,6 alpha-diphenyl glycoluril (Iodogen) Anal Biochem. 1981;117:136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- 18.Lubaroff DM, Canfield L, Feldbush TL, Bonney WW. R3327 adenocarcinoma of the Copenhagen rat as a model for the study of the immunologic aspects of prostate cancer. J Natl Cancer Inst. 1977;58:1677–1689. doi: 10.1093/jnci/58.6.1677. [DOI] [PubMed] [Google Scholar]
- 19.Demoy M, Minko T, Kopeckova P, Kopecek J. Time- and concentration-dependent apoptosis and necrosis induced by free and HPMA copolymer-bound doxorubicin in human ovarian carcinoma cells. J Control Release. 2000;69:185–196. doi: 10.1016/s0168-3659(00)00301-1. [DOI] [PubMed] [Google Scholar]
- 20.Kovar M, Kovar L, Subr V, Etrych T, Ulbrich K, Mrkvan T, Loucka J, Rihova B. HPMA copolymers containing doxorubicin bound by a proteolytically or hydrolytically cleavable bond: comparison of biological properties in vitro. J Control Release. 2004;99:301–314. doi: 10.1016/j.jconrel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Akporiaye ET, Hersh E. Clinical aspects of intratumoral gene therapy. Curr Opin Mol Ther. 1999;4:443–453. [PubMed] [Google Scholar]
- 22.Neyns B, Noppen M. Intratumoral gene therapy for non-small cell lung cancer: current status and future directions. Monaldi Arch Chest Dis. 2003;59:287–295. [PubMed] [Google Scholar]
- 23.Aigner KR, Gailhofer S, Kopp S. Regional versus systemic chemotherapy for advanced pancreatic cancer: a randomized study. Hepatogastroenterology. 1998;45:1125–1129. [PubMed] [Google Scholar]
- 24.Wientjes MG, Zheng JH, Hu L, Gan Y, Au JL. Intraprostatic chemotherapy: distribution and transport mechanisms. Clin Cancer Res. 2005;11:4204–4211. doi: 10.1158/1078-0432.CCR-04-1969. [DOI] [PubMed] [Google Scholar]
- 25.Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25:3825–3831. [PMC free article] [PubMed] [Google Scholar]
- 26.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–49. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase III trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerin C, Olivi A, Weingart JD, Lawson HC, Brem H. Recent advances in brain tumor therapy: local intracerebral drug delivery by polymers. Invest New Drugs. 2004;22:27–37. doi: 10.1023/b:drug.0000006172.65135.3e. [DOI] [PubMed] [Google Scholar]
- 29.Harrington KJ, Rowlinson-Busza G, Syrigos KN, Uster PS, Vile RG, Stewart JS. Pegylated liposomes have potential as vehicles for intratumoral and subcutaneous drug delivery. Clin Cancer Res. 2000;6:2528–2537. [PubMed] [Google Scholar]