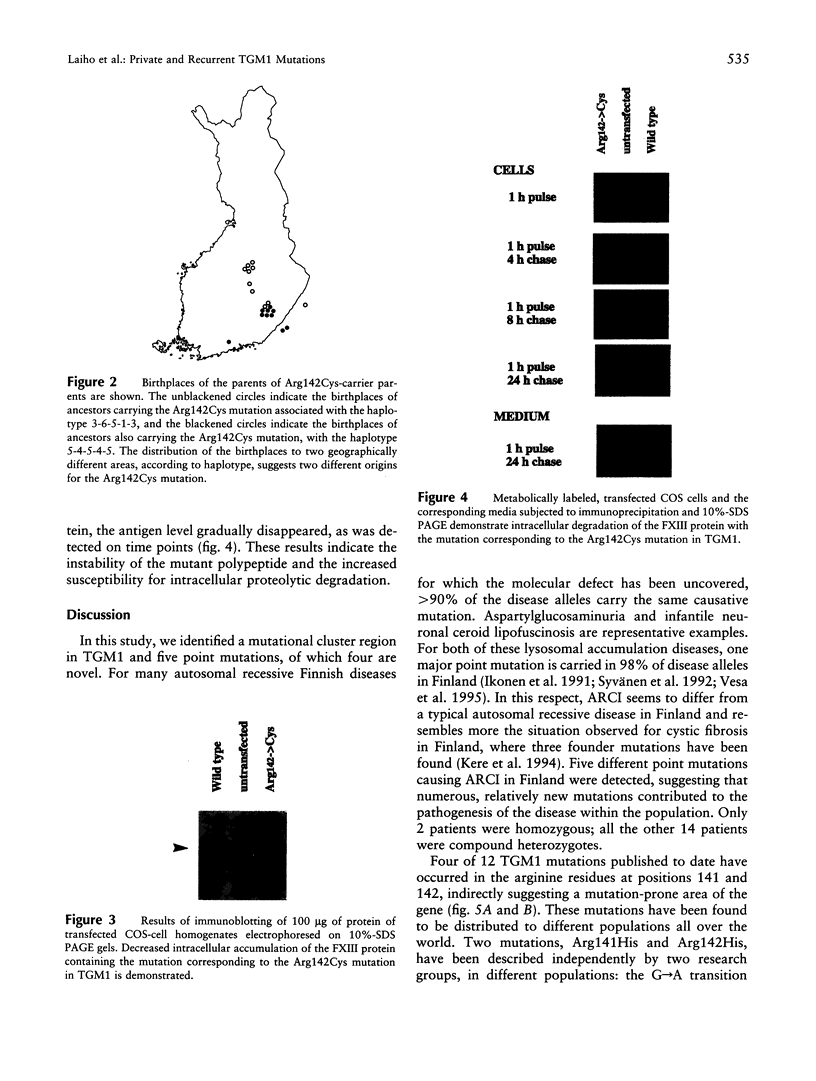
Abstract
Autosomal recessive congenital ichthyosis (ARCI) is a rare, heterogenous keratinization disorder of the skin, classically divided into two clinical subtypes, lamellar ichthyosis (LI) and nonbullous congenital ichthyosiformis erythroderma (CIE). Recently, strong evidence for the involvement of the transglutaminase 1 gene (TGM1) in LI has evolved. We have studied ARCI in the isolated Finnish population, in which recessive disorders are often caused by single mutations enriched by a founder effect. Surprisingly, five different mutations of TGM1 (Arg141His, Arg142Cys, Gly217Ser, Val378Leu, and Arg395Leu) were found in Finnish ARCI patients. In addition to affected LI patients, we also identified TGM1 mutations in CIE patients. Moreover, haplotype analysis of the chromosomes carrying the most common mutation, a C-->T transition changing Arg142 to Cys, revealed that the same mutation has been introduced twice in the Finnish population. In addition to this Arg142Cys mutation, three other mutations, in Arg141 and Arg142, have been described elsewhere, in other populations. These findings suggest that this region of TGM1 is more susceptible to mutation. The corresponding amino acid sequence is conserved in other transglutaminases, but, for example, coagulation factor XIII (FXIII) mutations do not cluster in this region. Protein modeling of the Arg142Cys mutation suggested disruption or destabilization of the protein. In transfection studies, the closely related transglutaminase FXIII protein with the corresponding mutation was shown to be susceptible to degradation in COS cells, further supporting evidence of the destabilizing effect of the Arg142Cys mutation in TGM1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeschlimann D., Paulsson M. Transglutaminases: protein cross-linking enzymes in tissues and body fluids. Thromb Haemost. 1994 Apr;71(4):402–415. [PubMed] [Google Scholar]
- Bale S. J., Doyle S. Z. The genetics of ichthyosis: a primer for epidemiologists. J Invest Dermatol. 1994 Jun;102(6):49S–50S. doi: 10.1111/1523-1747.ep12388591. [DOI] [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Ghadially R., Williams M. L., Hou S. Y., Elias P. M. Membrane structural abnormalities in the stratum corneum of the autosomal recessive ichthyoses. J Invest Dermatol. 1992 Dec;99(6):755–763. doi: 10.1111/1523-1747.ep12614489. [DOI] [PubMed] [Google Scholar]
- Hohl D., de Viragh P. A., Amiguet-Barras F., Gibbs S., Backendorf C., Huber M. The small proline-rich proteins constitute a multigene family of differentially regulated cornified cell envelope precursor proteins. J Invest Dermatol. 1995 Jun;104(6):902–909. doi: 10.1111/1523-1747.ep12606176. [DOI] [PubMed] [Google Scholar]
- Huber M., Rettler I., Bernasconi K., Frenk E., Lavrijsen S. P., Ponec M., Bon A., Lautenschlager S., Schorderet D. F., Hohl D. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science. 1995 Jan 27;267(5197):525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Baumann M., Grön K., Syvänen A. C., Enomaa N., Halila R., Aula P., Peltonen L. Aspartylglucosaminuria: cDNA encoding human aspartylglucosaminidase and the missense mutation causing the disease. EMBO J. 1991 Jan;10(1):51–58. doi: 10.1002/j.1460-2075.1991.tb07920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalanko A., Manninen T., Peltonen L. Deletion of the C-terminal end of aspartylglucosaminidase resulting in a lysosomal accumulation disease: evidence for a unique genomic rearrangement. Hum Mol Genet. 1995 Mar;4(3):435–441. doi: 10.1093/hmg/4.3.435. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kere J., Estivill X., Chillón M., Morral N., Nunes V., Norio R., Savilahti E., de la Chapelle A. Cystic fibrosis in a low-incidence population: two major mutations in Finland. Hum Genet. 1994 Feb;93(2):162–166. doi: 10.1007/BF00210603. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Chung S. I., Steinert P. M. Highly active soluble processed forms of the transglutaminase 1 enzyme in epidermal keratinocytes. J Biol Chem. 1995 Jul 28;270(30):18026–18035. doi: 10.1074/jbc.270.30.18026. [DOI] [PubMed] [Google Scholar]
- Mehrel T., Hohl D., Rothnagel J. A., Longley M. A., Bundman D., Cheng C., Lichti U., Bisher M. E., Steven A. C., Steinert P. M. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990 Jun 15;61(6):1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- Mikkola H., Syrjälä M., Rasi V., Vahtera E., Hämäläinen E., Peltonen L., Palotie A. Deficiency in the A-subunit of coagulation factor XIII: two novel point mutations demonstrate different effects on transcript levels. Blood. 1994 Jul 15;84(2):517–525. [PubMed] [Google Scholar]
- Mikkola H., Yee V. C., Syrjälä M., Seitz R., Egbring R., Petrini P., Ljung R., Ingerslev J., Teller D. C., Peltonen L. Four novel mutations in deficiency of coagulation factor XIII: consequences to expression and structure of the A-subunit. Blood. 1996 Jan 1;87(1):141–151. [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier L., Blanchet-Bardon C., Nguyen S., Prud'homme J. F., Dubertret L., Weissenbach J. Autosomal recessive lamellar ichthyosis: identification of a new mutation in transglutaminase 1 and evidence for genetic heterogeneity. Hum Mol Genet. 1995 Aug;4(8):1391–1395. doi: 10.1093/hmg/4.8.1391. [DOI] [PubMed] [Google Scholar]
- Parmentier L., Lakhdar H., Blanchet-Bardon C., Marchand S., Dubertret L., Weissenbach J. Mapping of a second locus for lamellar ichthyosis to chromosome 2q33-35. Hum Mol Genet. 1996 Apr;5(4):555–559. doi: 10.1093/hmg/5.4.555. [DOI] [PubMed] [Google Scholar]
- Paunio T., Kangas H., Kalkkinen N., Haltia M., Palo J., Peltonen L. Toward understanding the pathogenic mechanisms in gelsolin-related amyloidosis: in vitro expression reveals an abnormal gelsolin fragment. Hum Mol Genet. 1994 Dec;3(12):2223–2229. doi: 10.1093/hmg/3.12.2223. [DOI] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- Rice R. H., Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell. 1979 Nov;18(3):681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell. 1977 Jun;11(2):417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- Russell L. J., DiGiovanna J. J., Hashem N., Compton J. G., Bale S. J. Linkage of autosomal recessive lamellar ichthyosis to chromosome 14q. Am J Hum Genet. 1994 Dec;55(6):1146–1152. [PMC free article] [PubMed] [Google Scholar]
- Russell L. J., DiGiovanna J. J., Rogers G. R., Steinert P. M., Hashem N., Compton J. G., Bale S. J. Mutations in the gene for transglutaminase 1 in autosomal recessive lamellar ichthyosis. Nat Genet. 1995 Mar;9(3):279–283. doi: 10.1038/ng0395-279. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Schnieke A. SVpoly: a versatile mammalian expression vector. Nucleic Acids Res. 1990 May 11;18(9):2829–2829. doi: 10.1093/nar/18.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Harju L., Kontula K., Söderlund H. A primer-guided nucleotide incorporation assay in the genotyping of apolipoprotein E. Genomics. 1990 Dec;8(4):684–692. doi: 10.1016/0888-7543(90)90255-s. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Aalto-Setälä K., Kontula K., Söderlund H. Direct sequencing of affinity-captured amplified human DNA application to the detection of apolipoprotein E polymorphism. FEBS Lett. 1989 Nov 20;258(1):71–74. doi: 10.1016/0014-5793(89)81618-7. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Ikonen E., Manninen T., Bengtström M., Söderlund H., Aula P., Peltonen L. Convenient and quantitative determination of the frequency of a mutant allele using solid-phase minisequencing: application to aspartylglucosaminuria in Finland. Genomics. 1992 Mar;12(3):590–595. doi: 10.1016/0888-7543(92)90452-x. [DOI] [PubMed] [Google Scholar]
- Vesa J., Hellsten E., Verkruyse L. A., Camp L. A., Rapola J., Santavuori P., Hofmann S. L., Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995 Aug 17;376(6541):584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. Heterogeneity in autosomal recessive ichthyosis. Clinical and biochemical differentiation of lamellar ichthyosis and nonbullous congenital ichthyosiform erythroderma. Arch Dermatol. 1985 Apr;121(4):477–488. doi: 10.1001/archderm.121.4.477. [DOI] [PubMed] [Google Scholar]
- Yee V. C., Pedersen L. C., Le Trong I., Bishop P. D., Stenkamp R. E., Teller D. C. Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7296–7300. doi: 10.1073/pnas.91.15.7296. [DOI] [PMC free article] [PubMed] [Google Scholar]