Abstract
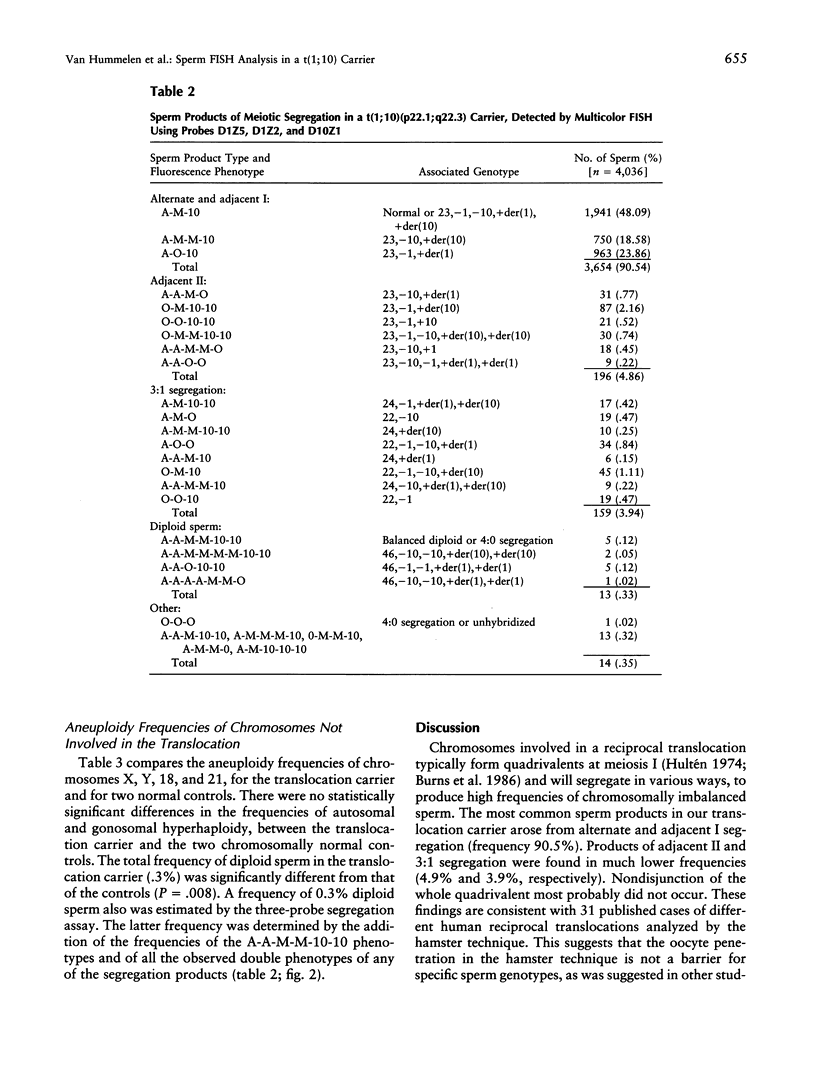
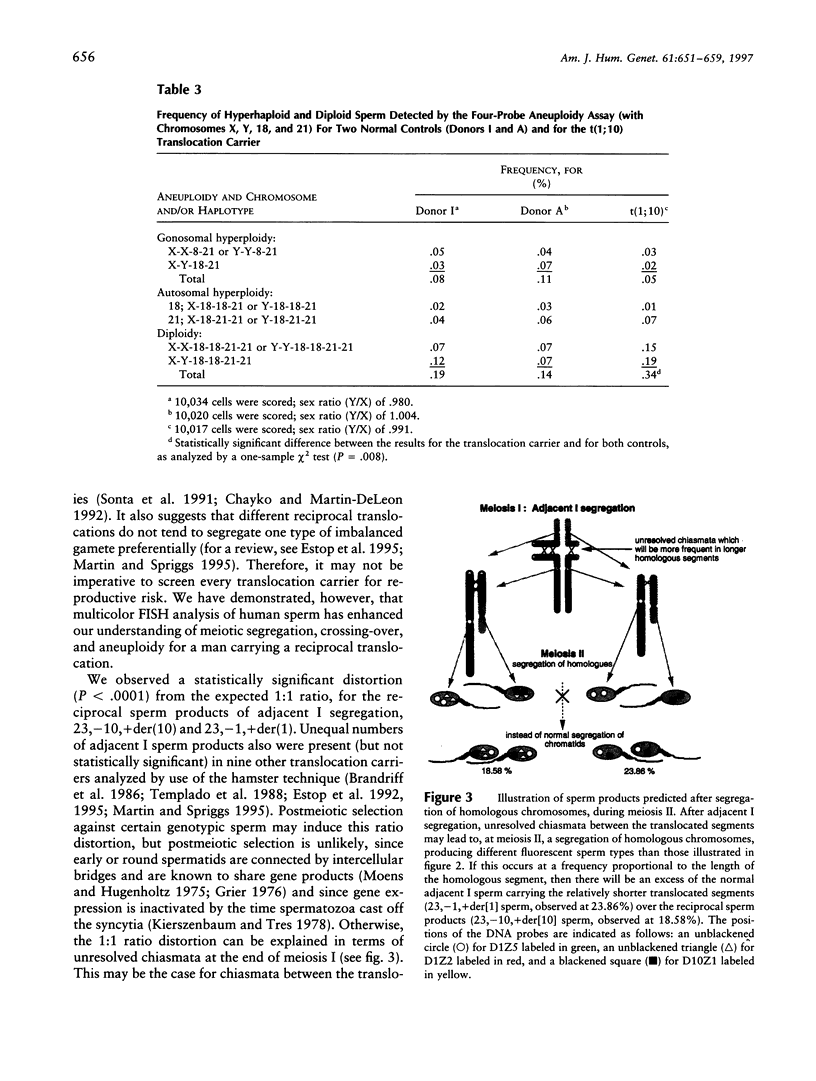
Meiotic segregation, recombination, and aneuploidy was assessed for sperm from a t(1;10)(p22.1;q22.3) reciprocal translocation carrier, by use of two multicolor FISH methods. The first method utilized three DNA probes (a telomeric and a centromeric probe on chromosome 1 plus a centromeric probe on chromosome 10) to analyze segregation patterns, in sperm, of the chromosomes involved in the translocation. The aggregate frequency of sperm products from alternate and adjacent I segregation was 90.5%, and the total frequency of normal and chromosomally balanced sperm was 48.1%. The frequencies of sperm products from adjacent II segregation and from 3:1 segregation were 4.9% and 3.9%, respectively. Reciprocal sperm products from adjacent I segregation deviated significantly from the expected 1:1 ratio (P < .0001). Our assay allowed us to evaluate recombination events in the interstitial segments at adjacent II segregation. The frequencies of sperm products resulting from interstitial recombination in chromosome 10 were significantly higher than those resulting from interstitial recombination in chromosome 1 (P < .006). No evidence of an interchromosomal effect on aneuploidy was found by use of a second FISH method that simultaneously utilized four chromosome-specific DNA probes to quantify the frequencies of aneuploid sperm for chromosomes X, Y, 18, and 21. However, a significant higher frequency of diploid sperm was detected in the translocation carrier than was detected in chromosomally normal and healthy controls. This study illustrates the advantages of multicolor FISH for assessment of the reproductive risk associated with translocation carriers and for investigation of the mechanisms of meiotic segregation of chromosomes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurias A., Prieur M., Dutrillaux B., Lejeune J. Systematic analysis of 95 reciprocal translocations of autosomes. Hum Genet. 1978 Dec 29;45(3):259–282. doi: 10.1007/BF00278725. [DOI] [PubMed] [Google Scholar]
- Brandriff B. F., Gordon L. A., Moore D., 2nd, Carrano A. V. An analysis of structural aberrations in human sperm chromosomes. Cytogenet Cell Genet. 1988;47(1-2):29–36. doi: 10.1159/000132500. [DOI] [PubMed] [Google Scholar]
- Brandriff B., Gordon L., Ashworth L. K., Littman V., Watchmaker G., Carrano A. V. Cytogenetics of human sperm: meiotic segregation in two translocation carriers. Am J Hum Genet. 1986 Feb;38(2):197–208. [PMC free article] [PubMed] [Google Scholar]
- Burns J. P., Koduru P. R., Alonso M. L., Chaganti R. S. Analysis of meiotic segregation in a man heterozygous for two reciprocal translocations using the hamster in vitro penetration system. Am J Hum Genet. 1986 Jun;38(6):954–964. [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T. Chiasma function. Cell. 1994 Jul 1;77(7):957–962. doi: 10.1016/0092-8674(94)90434-0. [DOI] [PubMed] [Google Scholar]
- Chayko C. A., Martin-DeLeon P. A. The murine Rb(6.16) translocation: alterations in the proportion of alternate sperm segregants effecting fertilization in vitro and in vivo. Hum Genet. 1992 Sep-Oct;90(1-2):79–85. doi: 10.1007/BF00210748. [DOI] [PubMed] [Google Scholar]
- Estop A. M., Levinson F., Cieply K., Vankirk V. The segregation of a translocation t(1;4) in two male carriers heterozygous for the translocation. Hum Genet. 1992 Jun;89(4):425–429. doi: 10.1007/BF00194315. [DOI] [PubMed] [Google Scholar]
- Estop A. M., Van Kirk V., Cieply K. Segregation analysis of four translocations, t(2;18), t(3;15), t(5;7), and t(10;12), by sperm chromosome studies and a review of the literature. Cytogenet Cell Genet. 1995;70(1-2):80–87. doi: 10.1159/000133997. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Hultén M. A. Chromosome in situ suppression hybridisation in human male meiosis. J Med Genet. 1992 Feb;29(2):98–102. doi: 10.1136/jmg.29.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier H. J. Sperm development in the teleost Oryzias latipes. Cell Tissue Res. 1976 May 26;168(4):419–431. doi: 10.1007/BF00215993. [DOI] [PubMed] [Google Scholar]
- Hultén M. Chiasma distribution at diakinesis in the normal human male. Hereditas. 1974;76(1):55–78. doi: 10.1111/j.1601-5223.1974.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Tres L. L. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed Proc. 1978 Sep;37(11):2512–2516. [PubMed] [Google Scholar]
- Kleckner N. Meiosis: how could it work? Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. H., Spriggs E. L. Sperm chromosome complements in a man heterozygous for a reciprocal translocation 46,XY,t(9;13)(q21.1;q21.2) and a review of the literature. Clin Genet. 1995 Jan;47(1):42–46. doi: 10.1111/j.1399-0004.1995.tb03920.x. [DOI] [PubMed] [Google Scholar]
- McKim K. S., Hawley R. S. Chromosomal control of meiotic cell division. Science. 1995 Dec 8;270(5242):1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Hugenholtz A. D. The arrangement of germ cells in the rat seminiferous tubule: an electron-microscope study. J Cell Sci. 1975 Dec;19(3):487–507. doi: 10.1242/jcs.19.3.487. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. Chromosome distribution: experiments on cell hybrids and in vitro. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 21;277(955):267–276. doi: 10.1098/rstb.1977.0017. [DOI] [PubMed] [Google Scholar]
- Robbins W. A., Baulch J. E., Moore D., 2nd, Weier H. U., Blakey D., Wyrobek A. J. Three-probe fluorescence in situ hybridization to assess chromosome X, Y, and 8 aneuploidy in sperm of 14 men from two healthy groups: evidence for a paternal age effect on sperm aneuploidy. Reprod Fertil Dev. 1995;7(4):799–809. doi: 10.1071/rd9950799. [DOI] [PubMed] [Google Scholar]
- Robbins W. A., Segraves R., Pinkel D., Wyrobek A. J. Detection of aneuploid human sperm by fluorescence in situ hybridization: evidence for a donor difference in frequency of sperm disomic for chromosomes 1 and Y. Am J Hum Genet. 1993 Apr;52(4):799–807. [PMC free article] [PubMed] [Google Scholar]
- Rousseaux S., Chevret E., Monteil M., Cozzi J., Pelletier R., Devillard F., Lespinasse J., Sèle B. Meiotic segregation in males heterozygote for reciprocal translocations: analysis of sperm nuclei by two and three colour fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;71(3):240–246. doi: 10.1159/000134118. [DOI] [PubMed] [Google Scholar]
- Sonta S., Yamada M., Tsukasaki M. Failure of chromosomally abnormal sperm to participate in fertilization in the Chinese hamster. Cytogenet Cell Genet. 1991;57(4):200–203. doi: 10.1159/000133146. [DOI] [PubMed] [Google Scholar]
- Spriggs E. L., Martin R. H. Analysis of segregation in a human male reciprocal translocation carrier, t(1;11) (p36.3;q13.1), by two-colour fluorescence in situ hybridization. Mol Reprod Dev. 1994 Jul;38(3):247–250. doi: 10.1002/mrd.1080380303. [DOI] [PubMed] [Google Scholar]
- Templado C., Navarro J., Benet J., Genescà A., Pérez M. M., Egozcue J. Human sperm chromosome studies in a reciprocal translocation t(2;5). Hum Genet. 1988 May;79(1):24–28. doi: 10.1007/BF00291704. [DOI] [PubMed] [Google Scholar]
- Van Hummelen P., Lowe X. R., Wyrobek A. J. Simultaneous detection of structural and numerical chromosome abnormalities in sperm of healthy men by multicolor fluorescence in situ hybridization. Hum Genet. 1996 Nov;98(5):608–615. doi: 10.1007/s004390050268. [DOI] [PubMed] [Google Scholar]
- Wyrobek A. J., Alhborn T., Balhorn R., Stanker L., Pinkel D. Fluorescence in situ hybridization to Y chromosomes in decondensed human sperm nuclei. Mol Reprod Dev. 1990 Nov;27(3):200–208. doi: 10.1002/mrd.1080270304. [DOI] [PubMed] [Google Scholar]


