Abstract
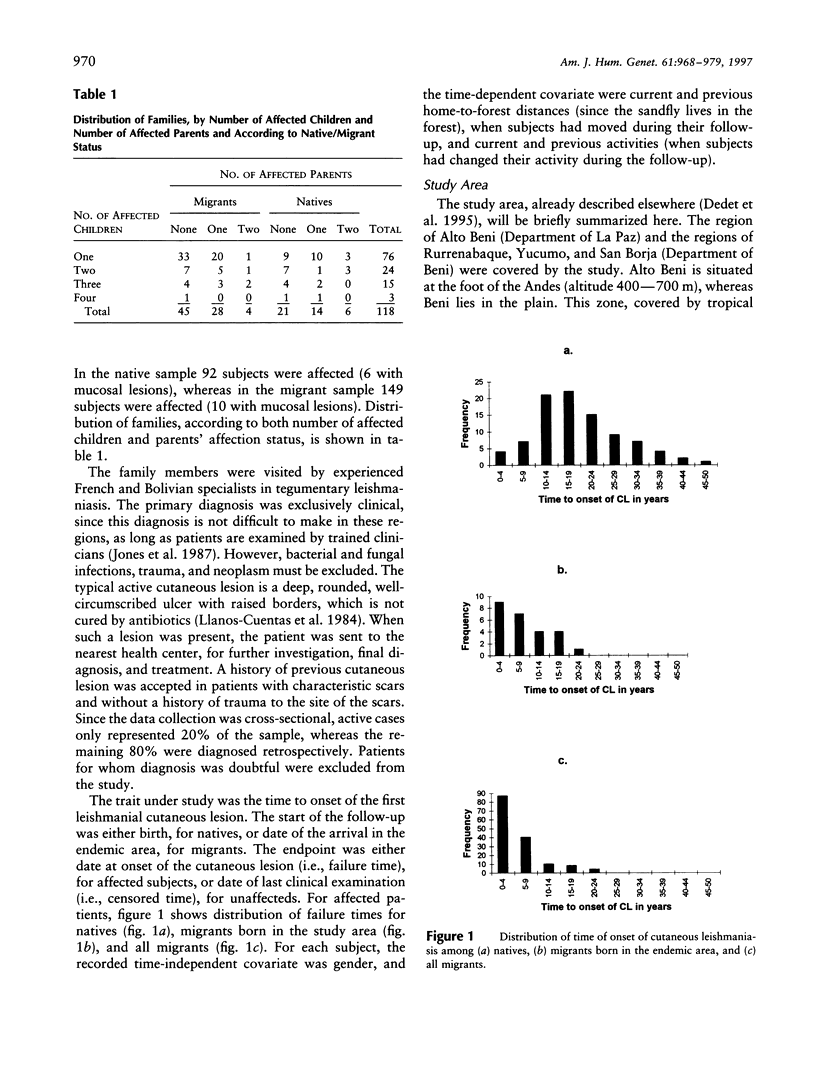
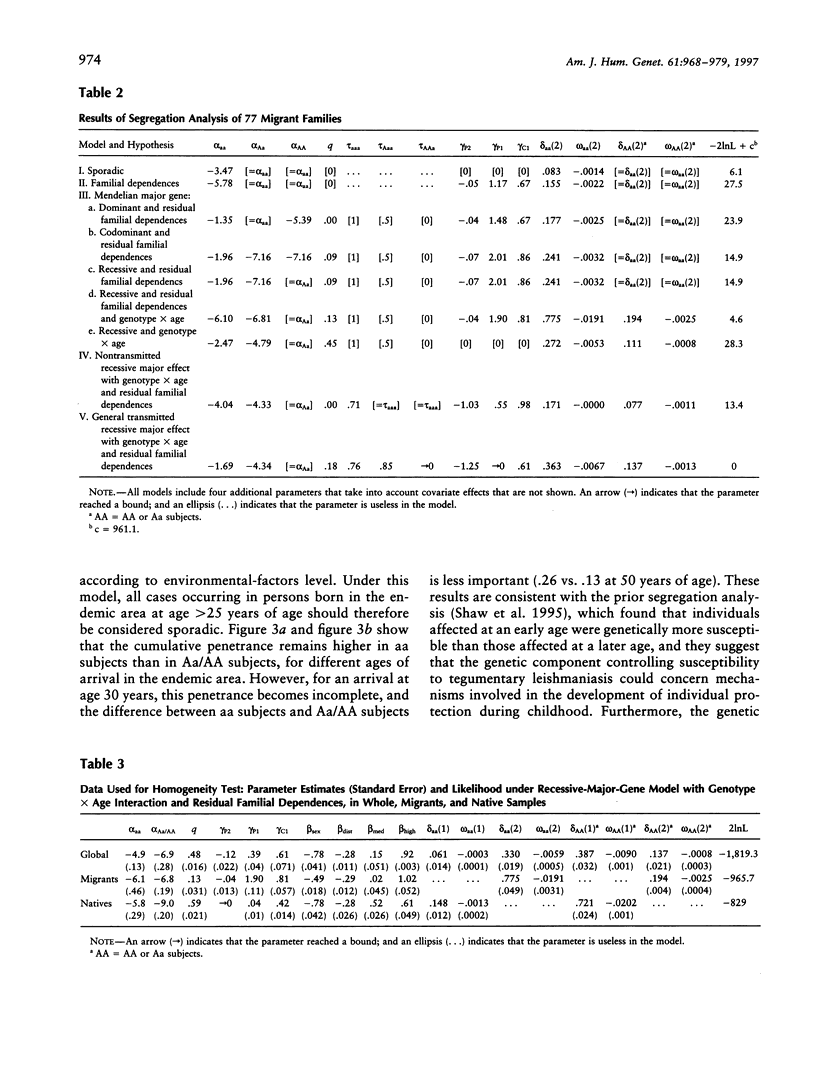
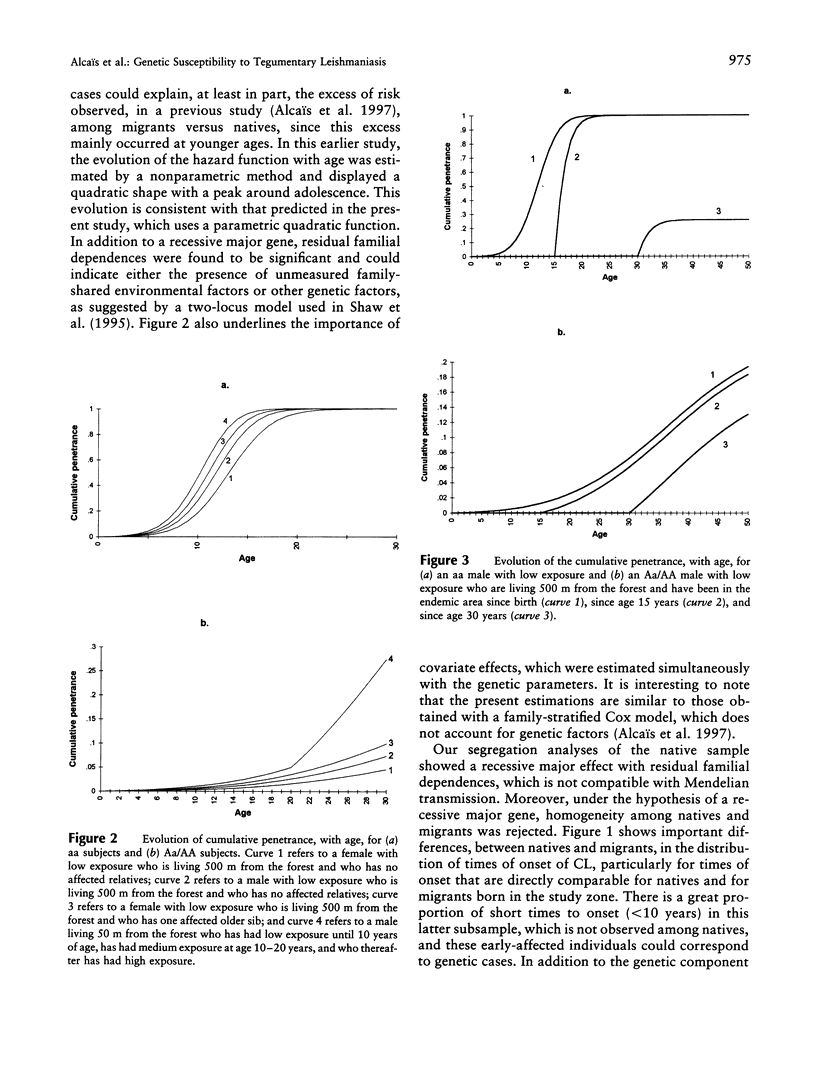
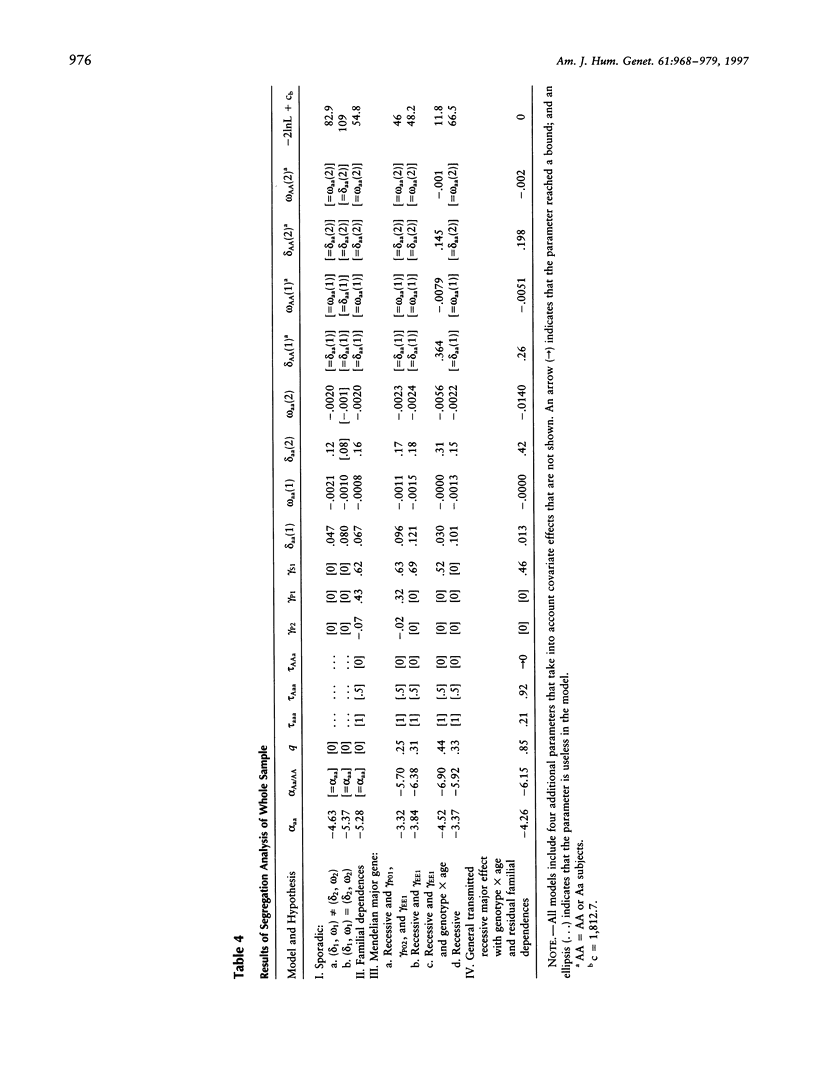
Tegumentary leishmaniasis due to Leishmania braziliensis is a parasitic disease that occurs in two stages after the infected sandfly bite: (1) a primary cutaneous lesion followed by (2) a secondary mucosal involvement generally resulting in severe facial deformities. In order to investigate the genetic and environmental factors involved in the development of the cutaneous lesion, a familial study was performed in a region of Bolivia in which the disease is endemic. Complete selection of 118 nuclear families (703 subjects, with 241 patients), each with at least one cutaneous affected subject, was achieved; 41 families were of native origin, and 77 (herein designated "migrant") recently had settled in the area. For the analysis, the trait under study was the time to onset of the primary cutaneous lesion. The start of the follow-up was birth, for native population, or date of arrival in the endemic area, for migrant population. Segregation analysis was performed by use of a model based on survival analysis methods that allows joint estimation of genetic and environmental effects and accounts for gene x covariate interactions. A significant effect of gender, home-forest distance, and forest-related activity was found. In the 77 migrant families there was evidence for a recessive major gene controlling the onset of the primary cutaneous lesion, with residual familial dependences and age x genotype interaction. Penetrance estimations show that young subjects are genetically more susceptible than older subjects, suggesting that this genetic component could concern mechanisms involved in the development of individual protection during childhood. There was also a significant genetic heterogeneity of the sample according to the native/migrant origin of the families, and no major-gene effect was found in the native subsample.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel L., Bonney G. E. A time-dependent logistic hazard function for modeling variable age of onset in analysis of familial diseases. Genet Epidemiol. 1990;7(6):391–407. doi: 10.1002/gepi.1370070602. [DOI] [PubMed] [Google Scholar]
- Alcais A., Abel L., David C., Torrez M. E., Flandre P., Dedet J. P. Risk factors for onset of cutaneous and mucocutaneous leishmaniasis in Bolivia. Am J Trop Med Hyg. 1997 Jul;57(1):79–84. doi: 10.4269/ajtmh.1997.57.79. [DOI] [PubMed] [Google Scholar]
- Antoine J. C. Biologie des interactions macrophages Leishmania. Pathol Biol (Paris) 1995 Mar;43(3):215–223. [PubMed] [Google Scholar]
- Barbier D., Demenais F., Lefait J. F., David B., Blanc M., Hors J., Feingold N. Susceptibility to human cutaneous leishmaniasis and HLA, Gm, Km markers. Tissue Antigens. 1987 Aug;30(2):63–67. doi: 10.1111/j.1399-0039.1987.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bonney G. E. Regressive logistic models for familial disease and other binary traits. Biometrics. 1986 Sep;42(3):611–625. [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Cabello P. H., Lima A. M., Azevedo E. S., Krieger H. Familial aggregation of Leishmania chagasi infection in northeastern Brazil. Am J Trop Med Hyg. 1995 Apr;52(4):364–365. doi: 10.4269/ajtmh.1995.52.364. [DOI] [PubMed] [Google Scholar]
- Cabrera M., Shaw M. A., Sharples C., Williams H., Castes M., Convit J., Blackwell J. M. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995 Nov 1;182(5):1259–1264. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier M., Govoni G., Vidal S., Kwan T., Groulx N., Liu J., Sanchez F., Skamene E., Schurr E., Gros P. Human natural resistance-associated macrophage protein: cDNA cloning, chromosomal mapping, genomic organization, and tissue-specific expression. J Exp Med. 1994 Nov 1;180(5):1741–1752. doi: 10.1084/jem.180.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Leishmania donovani. Hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978 Feb 1;147(2):515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C., Dimier-David L., Vargas F., Torrez M., Dedet J. P. Fifteen years of cutaneous and mucocutaneous leishmaniasis in Bolivia: a retrospective study. Trans R Soc Trop Med Hyg. 1993 Jan-Feb;87(1):7–9. doi: 10.1016/0035-9203(93)90398-a. [DOI] [PubMed] [Google Scholar]
- Davies C. R., Llanos-Cuentas E. A., Pyke S. D., Dye C. Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity. Epidemiol Infect. 1995 Apr;114(2):297–318. doi: 10.1017/s0950268800057964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedet J. P., Melogno R., Cardenas F., Valda L., David C., Fernandez V., Torrez M. E., Dimier-David L., Lyevre P., Villareal M. E. Rural campaign to diagnose and treat mucocutaneous leishmaniasis in Bolivia. Bull World Health Organ. 1995;73(3):339–345. [PMC free article] [PubMed] [Google Scholar]
- Demenais F. M., Laing A. E., Bonney G. E. Numerical comparisons of two formulations of the logistic regressive models with the mixed model in segregation analysis of discrete traits. Genet Epidemiol. 1992;9(6):419–435. doi: 10.1002/gepi.1370090605. [DOI] [PubMed] [Google Scholar]
- Demenais F. M. Regressive logistic models for familial diseases: a formulation assuming an underlying liability model. Am J Hum Genet. 1991 Oct;49(4):773–785. [PMC free article] [PubMed] [Google Scholar]
- Demenais F., Abel L. Robustness of the unified model to shared environmental effects in the analysis of dichotomous traits. Genet Epidemiol. 1989;6(1):229–234. doi: 10.1002/gepi.1370060140. [DOI] [PubMed] [Google Scholar]
- Demenais F., Lathrop M., Lalouel J. M. Robustness and power of the unified model in the analysis of quantitative measurements. Am J Hum Genet. 1986 Feb;38(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Elston R. C., Sobel E. Sampling considerations in the gathering and analysis of pedigree data. Am J Hum Genet. 1979 Jan;31(1):62–69. [PMC free article] [PubMed] [Google Scholar]
- Elston R. C., Stewart J. A general model for the genetic analysis of pedigree data. Hum Hered. 1971;21(6):523–542. doi: 10.1159/000152448. [DOI] [PubMed] [Google Scholar]
- Fine P. E. Immunogenetics of susceptibility to leprosy, tuberculosis, and leishmaniasis. An epidemiological perspective. Int J Lepr Other Mycobact Dis. 1981 Dec;49(4):437–454. [PubMed] [Google Scholar]
- Güler M. L., Gorham J. D., Hsieh C. S., Mackey A. J., Steen R. G., Dietrich W. F., Murphy K. M. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996 Feb 16;271(5251):984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Johnson W. D., Jr, Barretto A. C., Lago E., Badaro R., Cerf B., Reed S. G., Netto E. M., Tada M. S., Franca T. F. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis. 1987 Jul;156(1):73–83. doi: 10.1093/infdis/156.1.73. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Patel N. K., Blackwell J. M. Acquisition of cell-mediated immunity to Leishmania. II. LSH gene regulation of accessory cell function. Immunology. 1988 Sep;65(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- Lara M. L., Layrisse Z., Scorza J. V., Garcia E., Stoikow Z., Granados J., Bias W. Immunogenetics of human American cutaneous leishmaniasis. Study of HLA haplotypes in 24 families from Venezuela. Hum Immunol. 1991 Feb;30(2):129–135. doi: 10.1016/0198-8859(91)90081-j. [DOI] [PubMed] [Google Scholar]
- Marquet S., Abel L., Hillaire D., Dessein H., Kalil J., Feingold J., Weissenbach J., Dessein A. J. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat Genet. 1996 Oct;14(2):181–184. doi: 10.1038/ng1096-181. [DOI] [PubMed] [Google Scholar]
- Marsden P. D. Mucosal leishmaniasis ("espundia" Escomel, 1911). Trans R Soc Trop Med Hyg. 1986;80(6):859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- Petzl-Erler M. L., Belich M. P., Queiroz-Telles F. Association of mucosal leishmaniasis with HLA. Hum Immunol. 1991 Dec;32(4):254–260. doi: 10.1016/0198-8859(91)90088-q. [DOI] [PubMed] [Google Scholar]
- Pirmez C., Yamamura M., Uyemura K., Paes-Oliveira M., Conceiço-Silva F., Modlin R. L. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993 Apr;91(4):1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo S., Dimier-David L., David C., Lyevre P., Camacho C., Dedet J. P. Isoenzyme characterization of Leishmania braziliensis braziliensis isolates obtained from Bolivian and Peruvian patients. Trans R Soc Trop Med Hyg. 1992 Jul-Aug;86(4):388–391. doi: 10.1016/0035-9203(92)90231-z. [DOI] [PubMed] [Google Scholar]
- Rioux J. A., Lanotte G., Serres E., Pratlong F., Bastien P., Perieres J. Taxonomy of Leishmania. Use of isoenzymes. Suggestions for a new classification. Ann Parasitol Hum Comp. 1990;65(3):111–125. doi: 10.1051/parasite/1990653111. [DOI] [PubMed] [Google Scholar]
- Roberts M., Alexander J., Blackwell J. M. Influence of Lsh, H-2, and an H-11-linked gene on visceralization and metastasis associated with Leishmania mexicana infection in mice. Infect Immun. 1989 Mar;57(3):875–881. doi: 10.1128/iai.57.3.875-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Mock B. A., Blackwell J. M. Mapping of genes controlling Leishmania major infection in CXS recombinant inbred mice. Eur J Immunogenet. 1993 Oct;20(5):349–362. doi: 10.1111/j.1744-313x.1993.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Schurr E., Skamene E., Forget A., Gros P. Linkage analysis of the Bcg gene on mouse chromosome 1. Identification of a tightly linked marker. J Immunol. 1989 Jun 15;142(12):4507–4513. [PubMed] [Google Scholar]
- Shaw M. A., Davies C. R., Llanos-Cuentas E. A., Collins A. Human genetic susceptibility and infection with Leishmania peruviana. Am J Hum Genet. 1995 Nov;57(5):1159–1168. [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Walton B. C., Valverde L. Racial differences in espundia. Ann Trop Med Parasitol. 1979 Feb;73(1):23–29. doi: 10.1080/00034983.1979.11687222. [DOI] [PubMed] [Google Scholar]


