Abstract
Two flavonoids, genistein and apigenin, have been implicated as chemopreventive agents against prostate and breast cancers. However, the mechanisms behind their respective cancer-protective effects may vary significantly. The goal of this study was to determine whether the antiproliferative action of these flavonoids on prostate (DU-145) and breast (MDA-MB-231) cancer cells expressing only estrogen receptor (ER) β is mediated by this ER subtype. It was found that both genistein and apigenin, although not 17β-estradiol, exhibited antiproliferative effects and proapoptotic activities through caspase-3 activation in these two cell lines. In yeast transcription assays, both flavonoids displayed high specificity toward ERβ transactivation, particularly at lower concentrations. However, in mammalian assay, apigenin was found to be more ERβ-selective than genistein, which has equal potency in inducing transactivation through ERα and ERβ. Small interfering RNA-mediated downregulation of ERβ abrogated the antiproliferative effect of apigenin in both cancer cells but did not reverse that of genistein. Our data unveil, for the first time, that the anticancer action of apigenin is mediated, in part, by ERβ. The differential use of ERα and ERβ signaling for transaction between genistein and apigenin demonstrates the complexity of phytoestrogen action in the context of their anticancer properties.
Keywords: Phytoestrogens, genistein, ERα, apoptosis, cancer chemoprevention
Introduction
Flavonoids present in soy, fruits, and vegetables have been implicated as chemopreventive agents for a variety of cancers [1–3]. The best-studied flavonoid is genistein, an isoflavone abundant in soy. The beneficial effects of dietary soy are supported by epidemiological observations that countries with high soy consumption, such as China and Japan, have lower incidences of prostate and breast cancers than countries with little or no soy consumption [4]. In experimental models, dietary genistein reduces the incidence of prostate [5] and breast cancers [6,7]. Cellular and molecular mechanisms underpinning the anticancer effects of genistein cover a broad array of cellular processes, including suppression of cell growth, angiogenesis, oxidative stress, and tissue responses to estrogens [8]. Genistein is also recognized as a phytoestrogen because it binds to estrogen receptors (ERs) and exhibits both weak estrogenic and antiestrogenic activities.
Recently, the antitumor action of another dietary flavonoid, apigenin (4′,5,7-trihydroxyflavone), has received growing attention. It is abundantly present in leafy plants and vegetables (e.g., parsley, artichoke, basil, and celery) [9], but its production from manufacturers comes from extracts of dried flower heads of Matricaria chamomilla L. (http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Apigenin.pdf; accessed July 8, 2006). As a nutriceutic, apigenin is most widely used in treating anxiety and sleep disorders because it has been shown to possess sedative, antispasmodic, and spasmolytic actions [10]. The flavonoid also holds great promise as a chemopreventive agent for a variety of cancers. It exhibits significant activity against UV-induced DNA damage and thus may protect against skin cancer [11,12]. It inhibits the growth of a variety of human cancer cells, including leukemia and breast, colon, skin, thyroid, and prostate cancers [13,14]. Reported mechanisms associated with its antitumor action include induction of cell cycle arrest and apoptosis through a tumor necrosis factor-induced NFκB-mediated apoptosis pathway [14,15], attenuation of the phosphorylation of epidermal growth factor receptor and MAP kinase [16], promotion of HER-2/neu degradation [17], and activation of the intrinsic apoptosis pathway [18,19]. However, the likelihood that apigenin acts as an estrogen or antiestrogen has not been considered as a mechanism mediating its antitumor action.
It is now known that the actions of estrogens, antiestrogens, and phytoestrogens are mediated by two ER subtypes (ERα and ERβ) whose expression levels vary dramatically among different organs or cell types [20]. The two receptors regulate different sets of biologic functions and incite dissimilar responses within the same cell type or tissue. Furthermore, it has become apparent that the actions of these receptors vary dramatically, depending on whether they exist alone or together in a cell [21,22]. Because genistein and other phytoestrogens have been shown to preferentially use ERβs over ERαs as signaling mediators [23–26], it is reasonable to anticipate that apigenin exhibits a similar preference for estrogenicity.
The present study seeks to test the hypothesis that apigenin-induced cancer cell death is mediated by ERβ and neither by ERα nor androgen receptor. The prostate cancer cell line DU-145 [27,28] and the breast cancer cell line MDAMB-231 [29,30] were chosen as study models because they both express only ERβ. The growth-inhibitory action of apigenin on these cancer cell lines was examined in the presence or in the absence of small interfering RNA (siRNA)-mediated downregulation of the receptor. The transactivation activities of apigenin at the estrogen-responsive element (ERE), through ERβ, were compared to those mediated by ERα. Comparisons were also made between apigenin, 17β-estradiol (E2), ICI-182,780 (ICI), and genistein to elucidate the estrogenic properties of apigenin.
Materials and Methods
Reagents and Chemicals
Yeast synthetic dropout media were obtained from Clontech (Takara Bio, Palo Alto, CA). All steroids, phytoestrogens, and etoposide used in this study were purchased from Sigma (St. Louis, MO). The antiestrogen ICI was kindly supplied by Zeneca Pharmaceuticals (Cheshire, UK). The Beta-Glo Assay System was purchased from Promega (Madison, WI). DNA restriction enzymes were obtained from New England Biolabs, Inc. (Beverly, MA). Antibodies against human ERα (sc-8005) or ERβ (sc-8974) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Lines and Culture Conditions
Two human prostate cancer cell lines (DU-145 and PC-3), a breast cell line (MDA-MB-231), and an embryonic kidney cell line [human embryonic kidney (HEK) 293] were obtained from American Type Culture Collection (Manassas, VA). DU-145 and HEK293 cells were maintained in DMEM supplemented with 10% heat-inactivated fetal calf serum, nonessential amino acids, and penicillin/streptomycin (Invitrogen, Carlsbad, CA). PC-3 and MDA-MB-231 cells were maintained in DMEM/F12 or MEM-a medium, respectively, supplemented in the same fashion. Cells were maintained at 37°C and 5% CO2.
Cell Viability Assay
Cell viability assays were conducted in phenol red-free medium supplemented with 5% charcoal-stripped serum, nonessential amino acids, and penicillin/streptomycin. Cells were plated at 4 x 103 cells/well in 200 µl of phenol red-free medium in 96-well plates. Stock solutions of compounds in dimethyl sulfoxide (DMSO) were stored at 10 mM and mixed with fresh medium to achieve a final concentration of 10 nM E2, 1 µM ICI, or 20 µM genistein or apigenin. Cells were allowed to adhere for 24 hours, at which time the medium was removed and replaced with media containing one of the above agents. Control cultures received a medium containing the vehicle DMSO. Treatment was performed in triplicate and repeated at 48-hour intervals. On the sixth day, cell viability was determined by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; inner salt] assay, as described previously [31]. The medium was aspirated, and the cells were washed once with 200 µl of Hanks balanced salt solution. Ten microliters of MTS reagent (CellTiter 96 Aqueous One Solution Reagent; Promega) and 50 µl of medium were added to each well. Following 1 to 4 hours of incubation at 37°C and 5% CO2, absorbance was recorded by µQuant microplate reader (Biotek, Winooski, VT) at a wavelength of 490 nm.
Caspase-3 Assay
DU-145 or MDA-MB-231 cells were plated into six-well plates at 3 x 105 cells/well in 3 ml of phenol red-free medium and allowed to attach for 24 hours. Cells were treated with 10 nM E2, 1 µM ICI, and 20 µM etoposide, genistein, or apigenin. Control cultures were treated with vehicle alone (DMSO). Treatment time was 48 hours. The presence of apoptotic cells was determined by measuring caspase-3 using the BD ApoAlert Colorimetric Caspase-3 assay (Clontech), according to the manufacturer's instructions.
Knockdown of ERβ by Specific siRNA
Culture conditions for DU-145 (ERα- and ERβ+) and MDA-MB-231 (ERα- and ERβ+) have been described previously [27,29]. Cells were plated in 96-well or 6-well plates for MTS assay or RNA extraction, respectively, 1 day before transfection with siRNA oligonucleotides. Cells were transfected with 50 nM siRNA oligonucleotide using Lipofectamine2000 (Invitrogen), according to the manufacturer's protocol. siRNA against ERβ was purchased from Dharmacon's siGENOME SMART pool selection (Lafayette, CO) and was proven to knock down ERβ expression at the mRNA level by at least 75%. Negative control siRNA, an siCONTROL pool, and transfection siRNA control (siTOX) were included to ensure the specificity and transfection efficiency of siRNA. Twenty-four hours after transfection, cells were incubated with 10 or 20 µM apigenin or genistein for another 72 hours and subsequently analyzed for cytotoxicity with MTS assay. To correct for nonspecific toxic effects of siRNA, cell viability after treatments with a phytoestrogen (siESR2 + phytoestrogen or siCTL + phytoestrogen) was normalized against its respective no-phytoestrogen-treatment control (siESR2 alone or siCTL alone).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis of ERβ Transcript Levels
Total RNA was isolated from transfected cells with TRI reagent (Sigma), according to the manufacturer's protocol. The integrity of RNA was confirmed by denaturing gel, as described previously [32]. Total RNA (4 µg) from each sample was reverse-transcribed to cDNA by Superscript II reverse transcriptase (Invitrogen). Semiquantitative RT-PCR was performed using Platinum Taq polymerase (Invitrogen) with ERβ-specific primers [33]. The forward and reverse primers for β-actin have been described previously [27].
Construction of Yeast Strains
The yeast expression (YEpE10) and reporter (YEp-vERE) plasmids for ERα were kindly supplied by Dr. Tauseef Butt (LifeSensor, Inc., Malvern, PA). These plasmids were used to transform the protease-deficient yeast strain BJ2168 according to standard protocol [34]. This double-transformant yeast strain was grown in synthetic dropout medium (-TRP-URA). The yeast strain expressing ERβ in the presence of an estrogen-responsive reporter plasmid (YEp-vERE) has recently been described [33]. This double-transformant yeast strain was grown in synthetic dropout medium (-LEU-URA).
Yeast-Based Transcription Assays
To study the hormone responsiveness of ERα or ERβ, double yeast transformants carrying an expression plasmid for ERα or ERβ and a reporter plasmid (YEp-vERE) were selected for ligand-dependent transcriptional activity. Expression of ERβ was analyzed by Western blot analysis [35] using an N-terminal-specific H-150 polyclonal antibody (Santa Cruz Biotechnology). Because CUP1 promoter was moderately leaky in this experiment (data not shown), addition of copper was not necessary to induce ERβ expression. All selected transformants were grown in a synthetic dropout medium in a 96-well plate overnight at 30°C, either in the absence (control) or in the presence of steroids and phytoestrogens (0.01 nM–10 µM). A ligand-dependent transactivation assay was performed using the Beta-Glo Assay System (Promega), according to the manufacturer's protocol. Induction of the reporter gene (luciferase activity in relative light units) was measured by the Victor 2 system (Perkin Elmer, Wellesley, MA).
Transactivation Activity of ERα and ERβ in a Mammalian System
Mammalian expression vectors ERα and ERβ were gifts from Dr. Leigh C. Murphy (University of Manitoba, Winnipeg, Canada) [36]. The luciferase reporter plasmid carrying 3X vitellogenin ERE was kindly provided by Dr. Craig Jordan (Fox Chase Cancer Center, Philadelphia, PA) [37].
HEK293 cells at a density of 1.5 x 104 ml-1 were seeded onto a 24-well plate. Regular culture medium was replaced by phenol red-free DMEM with 5% charcoal-stripped serum. The cells were allowed to adopt an estrogen-free environment for 48 hours before transfection. Vectors expressing ERα or ERβ + ERE luciferase and β-galactosidase were transfected into the cells using Lipofectamine Plus (Invitrogen). After 24 hours of transfection, E2 (100 pM and 1 nM), ICI (10 nM and 1 µM), apigenin (100 nM and 1 µM), and genistein (100 nM and 1 µM) were applied to the culture. In separate experiments, cells were treated with E2, apigenin, or genistein in the absence or in the presence of the antiestrogen ICI. Luciferase reporter assay was performed as suggested in the Bright Glo Luciferase assay kit (Promega) to determine transactivation activity after 24 hours of treatment with hormones/compounds. Activities of β-galactosidase were measured by a β-gal assay kit (Promega) to normalize the transfection efficiency of each well.
Statistical Analysis
Data were expressed as mean ± SD. The statistical significance of the difference in means among treatment groups was determined with Systat software (Student version 6.0.1; SPSS, Chicago, IL) for one-way ANOVA followed by Tukey post hoc analyses. P < .05 was considered as a statistically significant difference between the two groups.
Results
Apigenin and Genistein Suppressed Cell Growth through Induction of Apoptosis in DU-145 and MDA-MB-231 Cells
The effects of E2 (10 nM), ICI (1 µM), genistein (20 µM), or apigenin (20 µM) on the growth of DU-145 and MDA-MB-231 in charcoal-stripped medium were examined. At the doses tested, E2 exerted no impact on the growth (Figure 1A and B) or the apoptosis (Figure 1C and D) of either cell lines, whereas ICI induced a small reduction in cell number and an increase in caspase-3 activation in DU-145 cells when compared with control vehicle (DMSO), but not in MDA-MB-231 cells. On the contrary, apigenin and genistein effectively suppressed the growth of both cell lines. Apigenin was more effective than genistein in suppressing DU-145 cell growth (Figure 1A) but exhibited potency equal to that of genistein in inhibiting MDA-MB-231 proliferation (Figure 1B). The growth inhibition induced by the two phytoestrogens paralleled their abilities to induce caspase-3 activation. Apigenin and genistein were equally effective in causing caspase-3 activation in both cell lines (Figure 1C and D).
Figure 1.
Effects of genistein or apigenin on the proliferation of (A) DU-145 and (B) MDA-MB-231 cells. Cells were treated with 10 nM E2, 1 µM ICI, 20 µM genistein, or 20 µM apigenin for 72 hours. Control (CTL) cultures were treated with solvent vehicle in a charcoal-stripped serum-supplemented medium. Cell viability was determined by MTS assay, as described in the Materials and Methods section. (C and D). Induction of relative caspase-3 activity by genistein or apigenin in (C) DU-145 and (D) MDA-MB-231 cells. Cells were treated with E2, ICI, genistein, and apigenin at the concentrations described above. E2 and etoposide serve as negative and positive control, respectively, for this experiment. Caspase-3 activities are normalized with respect to the control vehicle (DMSO). Twenty micromolars of etoposide was used as positive control. Data represent the averages (histograms) of three separate experiments, with the standard deviation indicated. aStatistically significant difference between the treatment group and the control group (control vehicle for A and B; E2 for C and D) at P < .05.
Differential Transcriptional Activation of ERα and ERβ in Response to Estrogens and Phytoestrogens in Yeast Cells
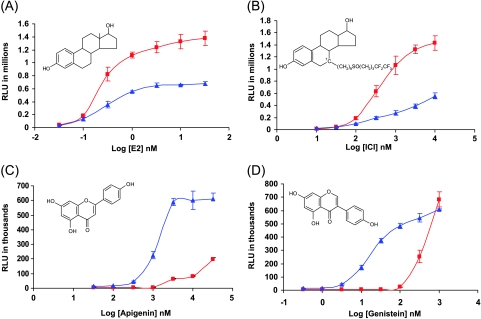
The transcriptional activities of E2, ICI, genistein, and apigenin through ERα and ERβ were evaluated in a yeast system. As expected, E2 was found to transactivate better through ERα than through ERβ (Figure 2A). Interestingly, although ICI is a well-recognized antagonist in mammalian cell studies, it behaved as an agonist in yeast cells, transactivatingmore effectively with ERα than with ERβ (Figure 2B). This finding is in agreement with findings in a previous report using yeast reporter assays [38]. In contrast to E2 or ICI, apigenin and genistein behaved as ERβ-selective ligands at low phytoestrogen concentrations. At higher ligand concentrations, they still elicited much higher transactivation activities through ERβ than through ERα (Figure 2C and D).
Figure 2.
Differential transcriptional activation of ERα and ERβ1 in yeast. Yeast strains harboring the expression vectors for ERα (red square) or ERβ1 (blue triangle) and the vitellogenin ERE reporter plasmid were incubated with an increasing concentration of E2 (A), ICI (B), apigenin (C), or genistein (D). After 24 hours of incubation at 30°C, Beta-Glo assays were performed. Luciferase activity (relative light units) was recorded by a luminometer (Victor 2 system). Each point represents an average of triplicates with standard deviations.
Differential Transactivation of ERα and ERβ in Response to Estrogens and Phytoestrogens in Mammalian Cells
The HEK293 cell line was chosen as a mammalian reporter assay system due to its low background as a transcription assay and the absence of endogenous ERα and ERβ expression [33]. When physiological concentrations of E2 (100 pM and 1 nM) were used, this ligand elicited potent dose-dependent transactivation responses through ERα (Figure 3A). In contrast, E2 elicited only low levels of transactivation through the ERβ. Contrary to results obtained in yeast systems, the antiestrogen ICI was inactive in these mammalian transcription assays regardless of its action being mediated by ERα or ERβ. In this regard, the results are consistent with the widely accepted notion that ICI is an estrogen antagonist for mammalian cells. The two phytoestrogens clearly exhibited agonistic actions in mammalian reporter assays. Apigenin was a weak agonist at a lower concentration (100 nM) compared to genistein, which achieved maximal transactivation through ERα and ERβ at the same concentration. At a higher concentration (1 µM), apigenin exhibited clear ERβ selectivity; it induced significantly higher transactivation through ERβ than through ERα. Thus, these data appear to corroborate those observed in yeast-based transcription assays, except for those related to ICI, which acts as an agonist in yeast assays. Importantly, all transcriptional activation activities induced by E2, apigenin, and genistein through ERβ could be attenuated efficiently by the coincubation of cell cultures with 1 µM ICI, indicating that they were mediated by ERβ (Figure 3B). Similar results were obtained when this experiment was conducted with ERα expressing HEK293 (data not shown).
Figure 3.
(A) Transcriptional activation of ERα and ERβ1 in mammalian cells. HEK293 cells were transfected with vectors expressing ERα (red bar) or ERβ1 (blue bar), β-galactosidase, and a vector carrying 3X ERE luciferase reporter using Lipofectamine Plus, as described in the Materials and Methods section. After 24 hours, cells were treated with E2, ICI, apigenin, genistein, or solvent vehicle (CTL) and subsequently analyzed for luciferase activity. Data represent three separate experiments with standard deviations. aStatistical significance between the treated group and untreated controls at P < .01. bNo significant difference between the two groups. (B) Effects of ICI on the ligand responsiveness of ERβ1 in HEK293 cells were seeded onto 24-well plates and transfected with ERβ1 expression vector, 3X ERE luciferase reporter, and β-galactosidase expression plasmids, as described above. Cells were treated with solvent vehicle (CTL), E2, genistein, or apigenin in the absence (black bar) or in the presence (white bar) of 1 µM ICI and subsequently analyzed for luciferase activity. Data represent three separate experiments with standard deviation.
ERβ Plays a Role in Apigenin-Induced Cancer Cell Growth Inhibition
The relative efficacy of siRNA against ERβ (ERβ siRNA), negative control siRNA (siCONTROL pool), and transfection siRNA control (siTOX) in knocking down ERβ transcripts in transfected cells was determined by RT-PCR. As shown in Figure 4A, ERβ transcripts were reduced by > 85% in DU-145 and MDA-MB-231 cells, whereas the other siRNA (siCONTROL pool and siTOX) did not affect the expression of ERβ transcripts when compared to cultures without siRNA transfection (control cultures were treated with Lipofectamine alone). Transfection efficiency in these experiments was estimated as 70%.
Figure 4.
(A) ERβ1 knockdown experiments in DU-145 and MDA-MB-231 cells. Cells were transfected with 50 nM siRNA against ERβ1 or scrambled siRNA (siCONTROL), as described in the Materials and Methods section. After 72 hours of incubation, total RNA was extracted and semiquantitative RT-PCR was performed using specific primers against ERβ1 and β-actin. Similar results were obtained from two separate experiments. (B–E) Antiproliferation effects of apigenin or genistein on ERβ1 knockdown DU-145 and MDA-MB-231 cells. siRNA against ERβ (siESR2)- and siRNA scrambled control (siCONTROL)-transfected cells were incubated with 10 or 20 µM apigenin (B and C) or genistein (D and E) for 72 hours and subsequently analyzed for cytotoxicity by MTS assay, as described in the Materials and Methods section. Control groups (Lipofectamine2000) without siRNA transfection were treated with vehicle (CTL). The viability of the cells with apigenin or genistein treatment (siESR2 + phytoestrogen or siCTL + phytoestrogen) was normalized to values obtained in cultures treated similarly but without phytoestrogens (siESR2 alone and siCTL alone). The cell viability in each treatment group was calculated as a percentage of the value found in untreated controls without siRNA transfection. Data represent the average of three separate experiments, with standard deviation indicated. aThe mean of the treated group was statistically different from that of untreated controls at P < .05. bNo significant difference between the treated group and the untreated control.
Subsequently, DU-145 and MDA-MB-231 cells were examined for cell viability after treatment with phytoestrogens. For both cell lines, apigenin (10 and 20 µM) induced a dose-dependent reduction in cell growth (Figure 4B and C), whereas genistein caused only cell growth inhibition at the high dose (20 µM) (Figure 4D and E). Importantly, the antiproliferation effects of apigenin on DU-145 and MDAMB-231 were effectively abrogated by transfecting the cells with ERβ siRNA (Figure 4B and C), but not by transfection of siCONTROL (Figure 4B and C), siTOX (data not shown), or ERα siRNA (data not shown). In contrast, genistein-induced cancer cell growth suppression was not reversed by ERβ siRNA transfection (Figure 4D and E).
Discussion
The two flavonoids genistein and apigenin have been studied extensively for their antitumorigenic activities in various cancers, including prostate and breast cancers [8,14,15,39,40]. Although many different mechanisms of action have been proposed [8,13,16–18,39–42], a connection between the anticancer effects of these flavonoids and ERβ has not been described. Several reports have demonstrated that interactions between ERα and ERβ could significantly alter the transactivation activities of each receptor and biologic outcome [21,22]. Therefore, we have chosen the two cancer cell lines DU-145 and MDA-MB-231, which express only ERβ [27,29], to compare the antiproliferative effects of two flavonoids. Furthermore, because transcriptional activities of a nuclear receptor could be markedly altered by post-translational protein modification and coregulator interaction in mammalian cells [43], we have evaluated the transcriptional potentials of these two flavonoids in yeast and mammalian reporter assays. Because yeast cells are devoid of transcription coregulators and most posttranslational protein modification pathways, basal transcriptional activities could be obtained for comparison with activities in a mammalian reporter system (HEK293). In this study, we found that the exposure of DU-145 or MDA-MB-231 cells to either flavonoid elicited suppression of cell growth and activation of caspase-3—a hallmark of apoptosis. In regard to the concentrations of genistein or apigenin used and the extent of growth inhibition/apoptosis induction in cancer cells, our findings were consistent with those reported by others [8,19,39,44–46]. For example, genistein has been reported to trigger apoptosis in breast cancer through calciumdependent and calpain/caspase-12-dependent pathways [47]. In both yeast and mammalian reporter assays, the two flavonoids were found to act as agonists at the ERE and to exhibit ERβ selectivity for transactivation when compared to E2, which transactivates most effectively through the ERα. Between the two flavonoids, apigenin was found to be more selective with transactivations by ERβ than was genistein, which was equally potent in eliciting transactivation through the two ER subtypes. The major contribution of our study, however, resides in the finding that the antiproliferative/proapoptotic effect of apigenin is apparently mediated by ERβ, whereas that of genistein, at least in these two cell models, does not involve the receptor. To the best of our knowledge, this study, which used siRNA knockdown of ERβ, is the first to demonstrate the involvement of this ER subtype in the antiproliferative/proapoptotic effect of apigenin.
In DU-145 cell cultures, apigenin-induced growth inhibition was noticeably greater than that induced by genistein, whereas in MDA-MB-231 cell cultures, both flavonoids exhibited comparable potency. Consistent with our previous observations, E2 was found to exert little effect on the growth of DU-145 cells, whereas ICI induced a modest inhibitory action [27]. The action of the antiestrogen on the prostate cancer cell line was shown to be dependent on ERβ [27] and may involve a crosstalk with the NFnB signaling pathway [48]. Morrissey et al. [18] suggested that apigenin-mediated apoptosis in DU-145 may not involve ERs. Their conclusion was reached based on the cotreatment of apigenin-exposed DU-145 cells with ICI and their observation of a lack of attenuation of the apigenin effect. Our findings offer a possible explanation for their observation. Because ICI could exhibit its antiproliferative/proapoptotic on DU-145 cells per se [27], the addition of ICI to apigenin-treated cells would unlikely block the proapoptotic effects of apigenin. In this study, our use of siRNA to specifically knock down ERβ proves to be a better approach to demonstrating the involvement of the receptor in apigenin action.
In MDA-MB-231 breast cancer cells, as expected [49], E2 did not stimulate cell growth and ICI had no action on proliferation/apoptosis. These findings are consistent with previously reported findings that the lack of ERα in this cell line makes it insensitive to estrogen stimulation in terms of cell proliferation. In contrast, both genistein and apigenin are effective antiproliferative/proapoptotic agents for this ERα- cell line. In the case of apigenin, its action was found to be mediated by ERβ in this study. If our observation in MDA-MB-231 could be extended to ERα- breast cancers, apigenin might have clinical utility in the chemoprevention of the recurrence of these cancers [50].
In the present study, we demonstrated that apigenin and genistein, when compared to E2, exhibit markedly different transactivation potencies through the two ER subtypes. E2 effectively induces ERα-mediated transcription but only triggers minimal transactivation through ERβ. In contrast, apigenin and genistein are excellent ERβ-mediated transactivators. This property of the two flavonoids is most noticeable in yeast reporter assays, which lack modulations from endogenous transcriptional coregulators or cofactors. Even in mammalian cell assays (HEK293 cells), both flavonoids are highly effective in eliciting ERβ-mediated transactivation. A key difference between the two resides in the strong selectivity of apigenin for ERβ-mediated transactivation, whereas genistein is equally effective in activating ERα-mediated and ERβ-mediated transcription. Collectively, findings from yeast and mammalian reporter assays suggest that apigenin is an ERβ-selective ligand, whereas genistein can activate both receptor subtypes. This conclusion is corroborated by a recent report that found genistein to have a higher binding affinity toward ERα than does apigenin [51].
The differences between these two flavonoids as phytoestrogens could be related to several key attributes that define the mode of estrogen action. Generally speaking, phytoestrogens have weak binding affinities for both ER subtypes, but they bind ERβ better than ERα [52]. However, a greater binding affinity of a phytoestrogen for a specific ER subtype does not always correlate with its ability to better transactivate gene expression through that receptor [53,54]. Other important factors that determine selectivity for an ER subtype include the ability of the phytoestrogen to create a highaffinity coregulator-binding pocket by the correct positioning of helix 12 within the ligand-binding domain of the ER-ligand complex [55]. In this regard, phytoestrogens have been shown to confer ERβ with coregulator-recruiting affinity higher than that of ERα [23,56]. Although most soy isoflavones, including genistein, are believed to exert their actions primarily through ERβ signaling [57,58], recent studies [59,60] have raised doubts about this assumption. Our data from HEK293 reporter assays support these doubts as genistein was found to be equally effective in eliciting transactivation through either ER subtype. This lack of selectivity of genistein for ERβ signaling may pose a limit to its use as a chemopreventive agent for breast cancer because its ERα activity may post concern for increasing the risk of recurrence of ERα breast cancers, undesirable uterotrophic activities, and thromboembolic disorders. It is well established that estrogen action on the uterus and liver is exclusively mediated by ERα signaling [61].
The most intriguing finding of the current study is that the siRNA-mediated knockdown of ERβ blocked only the growthinhibitory effect of apigenin—not that of genistein—on DU-145 and MDA-MB-231 cells. This finding suggests that the anticancer growth effect of apigenin—but not of genistein—involves ERβ. Indeed, pharmacological dosages of genistein have been shown to trigger cytotoxic activities through ER-independent pathways, such as inhibition of tyrosine kinase and topoisomerase [62]. Although apigenin has been shown to elicit pleiotropic effects on a variety of pathways that mediate antitumor actions [14–17,19], our report is the first one to associate it with ERβ signaling. More recently, genistein and apigenin have been demonstrated to act as estrogen agonists in ERα/β- MCF-7 and T47-D cells by acting through ERα [29]. Whether apigenin could suppress cell growth in other ER+ cancer cell lines remains to be determined as both DU-145 and MDA-MB-231 cell lines express only ERβ but not ERα [27–29]. It has been reported that ERα can heterodimerize with ERβ and alter its transactivation activity [21,22]. It is therefore logical to expect cancer cells that express both ER subtypes to respond to apigenin in a manner different from what has been demonstrated in DU-145 and MDA-MB-231 cells. It is surprising to find that the genistein-induced anticancer cell growth action in DU-145 and MDA-MB-231 cells was not affected by siRNA-induced downregulation of ERβ. Although the anticancer action of genistein on prostate and breast cancers has been widely reported, it remains uncertain whether it is mediated through ERβ. In this study, we showed that, at least in two cancer cell lines that express only ERβ, the anticancer effects of genistein are mediated through mechanisms not involving this receptor.
In summary, we have demonstrated the preferred usage of ERβ by apigenin as a mediator in suppressing the growth of DU-145 and MDA-MB-231 cells. Overall, apigenin, when compared to genistein, has a much stronger selectivity for ERβ than for ERα. Continued efforts placed on this area of research might provide important insights on the synthesis of highly selective ERβ agonists for anticancer intervention.
Acknowledgement
We thank Irving Chung for editing the manuscript.
Footnotes
This research was supported, in part, by National Institute of Health grants DK61084, CA112532, CA15776, and ES013071, and Department of Defense grant DAMD-W81XWH-04-1-0165.
References
- 1.Adlercreutz H. Epidemiology of phytoestrogens. Bailliere's Clin Endocrinol Metab. 1998;12:605–623. doi: 10.1016/s0950-351x(98)80007-4. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem Pharmacol. 2000;60:1051–1059. doi: 10.1016/s0006-2952(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths K, Denis L, Turkes A, Morton MS. Phytoestrogens and diseases of the prostate gland. Bailliere's Clin Endocrinol Metab. 1998;12:625–647. doi: 10.1016/s0950-351x(98)80008-6. [DOI] [PubMed] [Google Scholar]
- 4.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 5.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–6782. [PubMed] [Google Scholar]
- 6.Barnes S. Phytoestrogens and breast cancer. Bailliere's Clin Endocrinol Metab. 1998;12:559–579. doi: 10.1016/s0950-351x(98)80004-9. [DOI] [PubMed] [Google Scholar]
- 7.Constantinou AI, Krygier AE, Mehta RR. Genistein induces maturation of cultured human breast cancer cells and prevents tumor growth in nude mice. Am J Clin Nutr. 1998;68:1426S–1430S. doi: 10.1093/ajcn/68.6.1426S. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potencyselective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 10.Losi G, Puia G, Garzon G, de Vuono MC, Baraldi M. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur J Pharmacol. 2004;502:41–46. doi: 10.1016/j.ejphar.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 12.Birt DF, Mitchell D, Gold B, Pour P, Pinch HC. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997;17:85–91. [PubMed] [Google Scholar]
- 13.Khan TH, Sultana S. Apigenin induces apoptosis in Hep G2 cells: possible role of TNF-alpha and IFN-gamma. Toxicology. 2006;217:206–212. doi: 10.1016/j.tox.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Shukla S, Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol Cancer Ther. 2006;5:843–852. doi: 10.1158/1535-7163.MCT-05-0370. [DOI] [PubMed] [Google Scholar]
- 15.Shukla S, Gupta S. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes. Clin Cancer Res. 2004;10:3169–3178. doi: 10.1158/1078-0432.ccr-03-0586. [DOI] [PubMed] [Google Scholar]
- 16.Yin F, Giuliano AE, Law RE, Van Herle AJ. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001;21:413–420. [PubMed] [Google Scholar]
- 17.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 18.Morrissey C, O'Neill A, Spengler B, Christoffel V, Fitzpatrick JM, Watson RW. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 19.Way TD, Kao MC, Lin JK. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neu-overexpressing breast cancer cells. FEBS Lett. 2005;579:145–152. doi: 10.1016/j.febslet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod. 2005;73:866–871. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Singlechain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24:7681–7694. doi: 10.1128/MCB.24.17.7681-7694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol. 2005;19:1555–1568. doi: 10.1210/me.2004-0381. [DOI] [PubMed] [Google Scholar]
- 23.An J, Tzagarakis-Foster C, Scharschmidt TC, Lomri N, Leitman DC. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J Biol Chem. 2001;276:17808–17814. doi: 10.1074/jbc.M100953200. [DOI] [PubMed] [Google Scholar]
- 24.Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- 25.Mueller SO. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J Chromatogr B Anal Technol Biomed Life Sci. 2002;777:155–165. doi: 10.1016/s1570-0232(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 26.Patisaul HB. Phytoestrogen action in the adult and developing brain. J Neuroendocrinol. 2005;17:57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- 27.Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–3182. [PubMed] [Google Scholar]
- 28.Mobley JA, L'Esperance JO, Wu M, Friel CJ, Hanson RH, Ho SM. The novel estrogen 17alpha-20Z-21-[(4-amino)phenyl]-19-norpregna-1,3,5(10),20-tetraene-3,17beta-diol induces apoptosis in prostate cancer cell lines at nanomolar concentrations in vitro. Mol Cancer Ther. 2004;3:587–595. [PubMed] [Google Scholar]
- 29.Rousseau C, Nichol JN, Pettersson F, Couture MC, Miller WH., Jr ERbeta sensitizes breast cancer cells to retinoic acid: evidence of transcriptional crosstalk. Mol Cancer Res. 2004;2:523–531. [PubMed] [Google Scholar]
- 30.Vladusic EA, Hornby AE, Guerra-Vladusic FK, Lakins J, Lupu R. Expression and regulation of estrogen receptor beta in human breast tumors and cell lines. Oncol Rep. 2000;7:157–167. doi: 10.3892/or.7.1.157. [DOI] [PubMed] [Google Scholar]
- 31.Mobley JA, Leav I, Zielie P, Wotkowitz C, Evans J, Lam YW, L'Esperance BS, Jiang Z, Ho SM. Branched fatty acids in dairy and beef products markedly enhance alpha-methylacyl-CoA racemase expression in prostate cancer cells in vitro. Cancer Epidemiol Biomark Prev. 2003;12:775–783. [PubMed] [Google Scholar]
- 32.Syed V, Ulinski G, Mok SC, Ho SM. Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. J Natl Cancer Inst. 2002;94:617–629. doi: 10.1093/jnci/94.8.617. [DOI] [PubMed] [Google Scholar]
- 33.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER) β isoforms: a key to understanding ER-β signaling. Proc Natl Acad Sci USA. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salerno AJ, He Z, Goos-Nilsson A, Ahola H, Mak P. Differential transcriptional regulation of the apoAI gene by retinoic acid receptor homo- and heterodimers in yeast. Nucleic Acids Res. 1996;24:566–572. doi: 10.1093/nar/24.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng B, Lu B, Leygue E, Murphy LC. Putative functional characteristics of human estrogen receptor-beta isoforms. J Mol Endocrinol. 2003;30:13–29. doi: 10.1677/jme.0.0300013. [DOI] [PubMed] [Google Scholar]
- 37.Catherino WH, Jordan VC. Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Lett. 1995;92:39–47. doi: 10.1016/0304-3835(95)03755-l. [DOI] [PubMed] [Google Scholar]
- 38.Lyttle CR, Damian-Matsumura P, Juul H, Butt TR. Human estrogen receptor regulation in a yeast model system and studies on receptor agonists and antagonists. J Steroid Biochem Mol Biol. 1992;42:677–685. doi: 10.1016/0960-0760(92)90108-u. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factorkappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi T, Nakata T, Kuzumaki T. Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer Lett. 2002;176:17–23. doi: 10.1016/s0304-3835(01)00738-8. [DOI] [PubMed] [Google Scholar]
- 42.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 43.Hall JM, McDonnell DP. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv. 2005;5:343–357. doi: 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- 44.Magee PJ, McGlynn H, Rowland IR. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004;208:35–41. doi: 10.1016/j.canlet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem Pharmacol. 2006;72:681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Torkin R, Lavoie JF, Kaplan DR, Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 47.Sergeev IN. Genistein induces Ca2+-mediated, calpain/caspase-12-dependent apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2004;321:462–467. doi: 10.1016/j.bbrc.2004.06.173. [DOI] [PubMed] [Google Scholar]
- 48.Leung YK, Gao Y, Lau KM, Zhang X, Ho SM. ICI 182,780-regulated gene expression in DU145 prostate cancer cells is mediated by estrogen receptor-beta/NFkappaB crosstalk. Neoplasia. 2006;8:242–249. doi: 10.1593/neo.05853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight WA, III, Osborne CK, Yochmowitz MG, McGuire WL. Steroid hormone receptors in the management of human breast cancer. Ann Clin Res. 1980;12:202–207. [PubMed] [Google Scholar]
- 51.Seo HS, Denardo DG, Jacquot Y, Laios I, Vidal DS, Zambrana CR, Leclercq G, Brown PH. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99:121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 52.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 53.Graumann K, Breithofer A, Jungbauer A. Monitoring of estrogen mimics by a recombinant yeast assay: synergy between natural and synthetic compounds? Sci Total Environ. 1999;225:69–79. doi: 10.1016/s0048-9697(99)80018-7. [DOI] [PubMed] [Google Scholar]
- 54.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 55.Brooks SC, Skafar DF. From ligand structure to biological activity: modified estratrienes and their estrogenic and antiestrogenic effects in MCF-7 cells. Steroids. 2004;69:401–418. doi: 10.1016/j.steroids.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J Biol Chem. 2000;275:35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 57.Cassidy A, Griffin B. Phyto-oestrogens: a potential role in the prevention of CHD? Proc Nutr Soc. 1999;58:193–199. doi: 10.1079/pns19990025. [DOI] [PubMed] [Google Scholar]
- 58.McCarty MF. Isoflavones made simple—genistein's agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses. 2006;66:1093–1114. doi: 10.1016/j.mehy.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 59.Diel P, Smolnikar K, Schulz T, Laudenbach-Leschowski U, Michna H, Vollmer G. Phytoestrogens and carcinogenesis—differential effects of genistein in experimental models of normal and malignant rat endometrium. Hum Reprod. 2001;16:997–1006. doi: 10.1093/humrep/16.5.997. [DOI] [PubMed] [Google Scholar]
- 60.Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(3):349–353. doi: 10.1289/ehp.02110s3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saunders PT. Oestrogen receptor beta (ER beta) Rev Reprod. 1998;3:164–171. doi: 10.1530/ror.0.0030164. [DOI] [PubMed] [Google Scholar]
- 62.Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Ando S. Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]