Abstract
Glioblastomas are malignant brain tumors that are rarely curable, even with aggressive therapy (surgery, chemotherapy, and radiation). Glioblastomas frequently display loss of PTEN and/or epidermal growth factor receptor activation, both of which activate the PI3K pathway. This pathway can increase vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF)-1α expression. We examined the effects of two human immunodeficiency virus protease inhibitors, nelfinavir and amprenavir, which inhibit Akt signaling, on VEGF and HIF-1α expression and on angiogenesis. Nelfinavir decreased VEGF mRNA expression and VEGF secretion under normoxia. Downregulation of P-Akt decreased VEGF secretion in a manner similar to that of nelfinavir, but the combination of the two had no greater effect, consistent with the idea that nelfinavir decreases VEGF through the PI3K/Akt pathway. Nelfinavir also decreased the hypoxic induction of VEGF and the hypoxic induction of HIF-1α, which regulates VEGF promoter. The effect of nelfinavir on HIF-1α was most likely mediated by decreased protein translation. Nelfinavir's effect on VEGF expression had the functional consequence of decreasing angiogenesis in in vivo Matrigel plug assays. Similar effects on VEGFand HIF-1α expression were seen with a different protease inhibitor, amprenavir. Our results support further research into these protease inhibitors for use in future clinical trials for patients with glioblastoma multiformes.
Keywords: Nelfinavir, amprenavir, VEGF, HIF-1α, Akt
Introduction
Glioblastoma multiforme (GBM), the most common brain tumor in adults, remains a difficult therapeutic challenge. GBMs are infiltrative high-grade gliomas that are associated with dismal survival in spite of aggressive therapy, including surgery, radiotherapy, and temozolomide [1]. For this reason, novel strategies, including antiangiogenic therapies, are being employed in the treatment of these tumors [2,3]. These strategies are motivated by the fact that glioblastomas often express very high levels of vascular endothelial growth factor (VEGF), a key mediator of blood vessel growth [4,5]. In other solid malignancies, the anti-VEGF monoclonal antibody bevacizumab has been shown to prolong survival when used in combination with chemotherapy [6].
A key stimulus for increased VEGF expression in glioblastomas is hypoxia, which is prevalent in these tumors [7,8] and leads to stabilization and increased expression of the a subunit of the transcription factor hypoxia-inducible factor (HIF)-1 [9]. HIF-1α heterodimerizes with HIF-1β, whose level does not vary with oxygen concentration, to transactivate an array of genes, many of which are involved in angiogenesis, glucose transport and metabolism, and tumor invasion and metastasis [8,9]. In a number of tumor xenograft models, decreased HIF-1α expression is associated with slower growth [10–12]. Knockout of HIF-1α has been reported to decrease in vitro growth even under normoxic conditions [13]. In some solid tumors, there is a correlation between high levels of HIF-1α and worse clinical outcome [14–16]. There is increasing expression of HIF-1α with increasing glioma grade, which also correlates with worsening prognosis [17]. For these reasons, many feel that both HIF-1α and VEGF are excellent targets for cancer therapy [2,8,9].
The PI3K pathway is commonly activated in glioblastomas, often by PTEN mutation but also possibly by epidermal growth factor receptor overexpression or activation by mutations [18,19]. Studies from our laboratory [20,21] and others [22,23] have confirmed a link between PI3K/Akt pathway activation and increased VEGF and HIF-1α expression. Recently, it has been shown that protease inhibitors, such as nelfinavir, currently used to treat human immunodeficiency virus (HIV) patients can radiosensitize tumor cells, possibly through inhibition of PI3K/Akt signaling [24]. Therefore, we were interested in testing whether these compounds could inhibit VEGF and HIF-1α expression in glioblastomas. We performed studies to examine the effects of two of these HIV protease inhibitors, nelfinavir and amprenavir, on VEGF and HIF-1α expression in vitro and on angiogenesis in vivo.
Materials and Methods
Tissue Culture and Reagents
U87MG was obtained from the American Type Culture Collection (Rockville, MD). U251MG was obtained from the Brain Tumor Research Center Tissue Bank at the University of California San Francisco (San Francisco, CA). Both cell lines were cultured in Dulbecco's modified Eagle's medium (4500 mg/l glucose; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and grown in an incubator containing 5% carbon dioxide and 21% oxygen. U87/PTEN doxycycline-inducible cells were a gift from M. Georgescu (M. D. Anderson Cancer Center, Houston, TX) [25]. These cells were cultured in the same medium used for U87MG cells, but with G418 (400 µg/ml) and blasticidin (2 µg/ml) added.
Hypoxic conditions were established as described previously [26,27]. Cells were plated onto 60-mm Permanox dishes (Munc, Rochester, NY) that were permeable to oxygen and were allowed to attach overnight. Immediately before the induction of hypoxia, the medium was replaced with 2 ml of fresh HEPES-buffered medium. Each dish was sealed in an aluminum chamber, and pO2 was decreased to the desired level by using a series of precision evacuations followed by replacement with nitrogen (gas exchange). After warming, the chambers were shaken continuously at 37°C to ensure that pO2 in the culture medium was in equilibrium with pO2 in the gas phase.
Northern Blot Analysis
Northern blot analysis was performed as described previously [21].
Protein Extraction and Western Blot Analysis
For details regarding protein isolation, gel electrophoresis, and Western blot analysis, see Pore et al. [28]. The following antibodies were used: monoclonal anti-phospho-Akt antibody that recognizes P-S473 (New England Biolabs, Ipswich, MA), anti-Akt antibody (New England Biolabs), anti-HIF-1α antibody (clone H1α67; Novus Biologicals, Littleton, CO) at 1:1000 dilution, and anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO) at 1:1000 dilution. The secondary antibody used for these blots was a goat anti-mouse antibody (BioRad, Hercules, CA). Antibody binding was detected by chemiluminescence using an ECL kit (Amersham Pharmacia, Piscataway, NJ).
VEGF Enzyme-Linked Immunosorbent Assay (ELISA)
An aliquot of conditioned medium was removed for storage at -80°C. VEGF protein concentration in the medium was determined by ELISA using a commercial kit (R&D Systems, Minneapolis, MN).
In Vivo Study of Angiogenesis Using Matrigel Plug Assay
Pathogen-free female Ncr-nu/nu mice were obtained from Taconic Industries (Germantown, NY) and housed in animal facilities of the University Laboratory Animal Resources and the Institute for Human Gene Therapy of the University of Pennsylvania (Philadelphia, PA). All experiments were carried out in accordance with the guidelines of the University Institutional Animal Care and Use Committee. Angiogenesis was measured in growth factor-free Matrigel (Collaborative Biomedical Products, Inc., Bedford, MD). Matrigel plugs (500 µl) containing 2 x 106 cells of each cell line were injected subcutaneously into the right and left sides of 4- to 8-week-old female BALB/c nude mice at sites lateral to the abdominal midline. As negative control, Matrigel with 100 µl of phosphate-buffered saline (PBS) was injected in a similar manner. All measurements were made in triplicate. Animals were sacrificed 5 days after Matrigel injection. Matrigel plugs were recovered and photographed immediately. Plugs were then dispersed in PBS and incubated overnight at 4°C. Using Drabkin's solution (Sigma-Aldrich), hemoglobin levels were determined according to the manufacturer's instructions. Hemoglobin level was calculated from a standard hemoglobin curve.
Statistical Analysis
Two-sided Student's t test was employed to compare the means between two groups (i.e., hemoglobin levels in Matrigel plugs between control and nelfinavir-treated mice).
Results
Nelfinavir Downregulates VEGF and HIF-1 Expression through Inactivation of PI3K/Akt Pathways
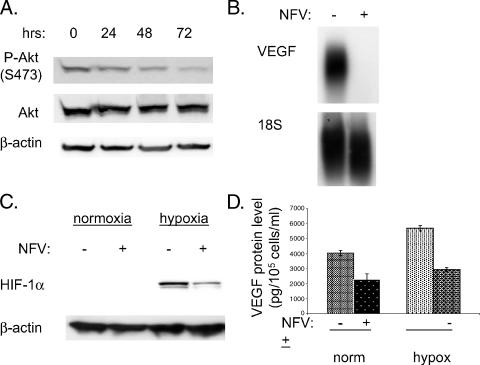
U87MG cells activate the PI3K/Akt pathway through loss of PTEN [29]. Nelfinavir inhibited Akt phosphorylation at serine 473 in human glioblastoma U87MG cells (Figure 1A). Phospho-Akt levels had decreased by 24 hours and had almost completely disappeared by 72 hours, whereas total Akt and b-actin levels were unchanged. Because the PI3K pathway regulates VEGF expression, we investigated the effect of nelfinavir on this. We found that nelfinavir dramatically decreases VEGF mRNA expression (Figure 1B). In addition to the effects of nelfinavir on VEGF expression under normoxia, the drug also blunted the induction of HIF-1α in response to hypoxia (Figure 1C). Likewise, nelfinavir also decreased VEGF secretion under normoxic and under hypoxic conditions, as determined by ELISA (Figure 1D).
Figure 1.
Nelfinavir decreases VEGF expression. (A) U87MG cells were treated with nelfinavir (NFV) (15 µM) for various lengths of time, as indicated. Then cells were harvested, and Western blot analysis was performed. The membrane was probed for phospho-Akt (S473), then subsequently reprobed for total Akt and β-actin (loading control). (B) U87MG cells were treated with nelfinavir for 24 hours (NFV); thereafter, cells were harvested for RNA, and Northern blot analysis was performed for VEGF and 18S (loading control). (C) U87MG cells were treated with nelfinavir (15 µM) for 24 hours, then cells were exposed to hypoxia (0.2% oxygen). Three hours later, cells were harvested for protein, and Western blot analysis was performed with HIF-1α antibody. Subsequently, membranes were reprobed for β-actin (loading control). (D) Cell culture medium was sampled 30 hours after nelfinavir treatment and/or hypoxia (0.2% O2). VEGF protein levels were determined by ELISA and normalized to the number of cells in each dish.
Nelfinavir Decreases Angiogenesis In Vivo
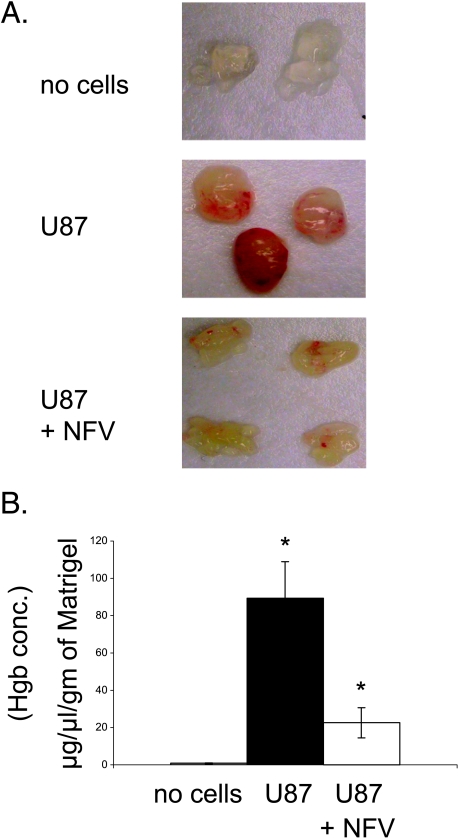
To determine whether nelfinavir-induced decrease in VEGF secretion in vitro had a functional consequence, we performed in vivo Matrigel assays. U87MG cells were placed into Matrigel plugs, which were implanted subcutaneously into nude mice. Five days later, the plugs were excised and evaluated for hemoglobin content. Nelfinavir decreased angiogenesis by visual inspection and hemoglobin measurement (Figure 2, A and B).
Figure 2.
Nelfinavir inhibits in vivo angiogenesis. (A) Matrigel mixture containing U87MG cells was injected subcutaneously into nude mice at sites lateral to the abdominal midline. Four mice were given feeds containing nelfinavir (40 mg/kg per day), and another three mice were given feeds without nelfinavir. As negative control, Matrigel containing 100 µl of PBS was injected into two mice. Five days later, the animals were sacrificed, Matrigel plugs were recovered and photographed immediately. Each discrete mass represents a plug removed from a different animal. (B) The relative level of hemoglobin present in each plug was determined using a commercially available kit. The hemoglobin level normalized to the weight of each Matrigel plug is plotted on the y-axis. *The comparison of mean hemoglobin values between these two groups (control and nelfinavir-treated mice injected with Matrigel plugs containing U87MG cells) was statistically significant at P < .01 (two-sided Student's t test).
Nelfinavir Downregulates HIF-1α through Inhibition of Protein Synthesis
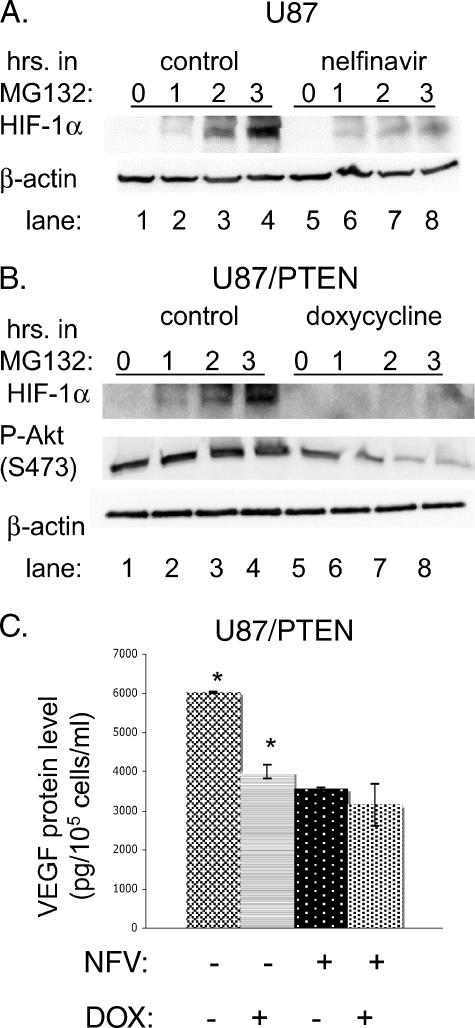
We wished to determine the mechanism by which nelfinavir decreased HIF-1α protein levels. HIF-1α undergoes rapid degradation by the proteasome under normoxic conditions [30,31]. Treatment of cells with the proteasomal inhibitor MG132 led to accumulation of HIF-1α under normoxia, as expected. However, pretreatment with nelfinavir prevented HIF-1α accumulation in the presence of MG132 (Figure 3A, cf. lanes 3 and 7 or lanes 4 and 8), suggesting that nelfinavir interfered with HIF-1α synthesis rather than with degradation. However, nelfinavir did not alter the level of HIF-1α mRNA (data not shown), thus indicating that it acted at the translational or the posttranslational level. Similar results were obtained by downregulating P-Akt expression in U87MG cells. To do this, we used a derivative cell line engineered such that addition of doxycycline induces wild-type PTEN [25]. We confirmed that P-Akt was downregulated in response to doxycycline (Figure 3B). Figure 3B shows that, when P-Akt was downregulated, the accumulation of HIF-1α in the presence of MG132 was impaired (compare lanes 3 and 7 or lanes 4 and 8). These results suggest that inactivation of the PI3K/Akt pathway by PTEN decreases HIF-1α protein synthesis in the same way that nelfinavir does and is consistent with the idea that inhibition of HIF-1α expression by nelfinavir occurs through the PI3K pathway.
Figure 3.
Nelfinavir decreases HIF-1α expression through alteration of protein translation.(A) U87MG cells were treated with 15 µM nelfinavir for 24 hours, then cells were exposed to the proteasome inhibitor MG132 (10 µM) for various durations of time, as indicated. (B) U87/PTEN-inducible cells were treated with doxycycline (2 µg/ml) for 16 hours, then cells were exposed to the proteasome inhibitor MG132 (10 µM) for various time periods, as indicated. For both (A) and (B), cells were harvested for protein, and Western blot analysis was performed for HIF-1α, P-Akt, and β-actin. (C) U87MG/PTEN cells were treated with doxycycline (DOX) for 16 hours, then cells were further treated for an additional 24 hours with nelfinavir (NFV) or control carrier (ethanol). Then the culture medium was sampled to determine VEGF protein levels by ELISA and was normalized to the number of cells in each dish. *The comparison of ELISA values between these two groups (control and doxycycline-treated U87/PTEN cells) was statistically significant at P < .01 (two-sided Student's t test). Comparisons between control and nelfinavir-treated or nelfinavir + doxycycline-treated cells were also statistically significant (P < .01).
To examine whether nelfinavir's ability to downregulate P-Akt and VEGF expression lie in a common pathway, we performed an epistasis-type analysis. Decreasing P-Akt levels in U87/PTEN cells by adding doxycycline decreased VEGF secretion to a similar extent as did the addition of nelfinavir; however, the combination of the two did not have an additive effect (Figure 3C). This suggests that nelfinavir decreases VEGF secretion through the Akt pathway.
Protease Inhibitor Amprenavir Inhibits VEGF and HIF-1 Expression in Glioblastoma Cells But Not in Normal Human Astrocytes
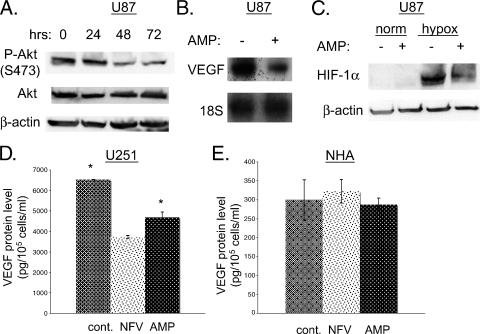
We tested another HIV protease inhibitor, amprenavir, which has been reported to inhibit Akt phosphorylation [24]. We confirmed that this drug inhibited the phosphorylation of Akt at serine 473 in U87MG cells (Figure 4A). Phospho-Akt levels had substantially decreased by 72 hours, whereas total Akt and b-actin levels were unchanged. Amprenavir, similar to nelfinavir, also decreased VEGF mRNA (Figure 4B) and protein expression (data not shown). Amprenavir also decreased the induction of HIF-1α in response to hypoxia (Figure 4C).
Figure 4.
Amprenavir inhibits the expression of HIF-1α and VEGF. (A) U87MG cells were treated with 15 µM amprenavir (AMP) for various lengths of time, as indicated. Then cells were harvested, and Western blot analysis was performed. The membrane was probed for P-Akt (S473) and total Akt, and then was reprobed for β-actin (loading control). (B) U87MG cells were treated with amprenavir for 24 hours; thereafter, cells were harvested for RNA, and Northern blot analysis was performed for VEGF and 18S (loading control). (C) U87MG cells were treated with amprenavir (15 µM) for 24 hours, then cells were exposed to hypoxia (0.2% oxygen). Three hours later, cells were harvested, and Western blot analysis was performed for HIF-1α. Membranes were then reprobed for β-actin (loading control). (D and E) U251MG or NHA cells were treated with nelfinavir (NFV) or amprenavir. Thirty hours later, the cell culture medium was collected, and VEGF protein levels were determined by ELISA and normalized to the number of cells in each dish. *The comparison of ELISA values between these two groups (control and amprenavir-treated U251MG cells) was statistically significant at P < .01 (two-sided Student's t test). The comparison between control and NFV-treated U251MG cells was also statistically significant (P < .01).
To generalize our findings, we used U251MG, another GBM cell line with increased P-Akt levels secondary to PTEN mutation [29]. In this cell line, both amprenavir and nelfinavir decreased VEGF secretion. In contrast, in immortalized human astrocytes (NHA) [32], which have wild-type PTEN and express very little VEGF, neither nelfinavir nor amprenavir, had any effect on VEGF expression.
Discussion
U87MG is a commonly used glioblastoma cell line that has been well-characterized. It displays loss of PTEN, leading to activation of the PI3K/Akt pathway. In vitro studies show these cells to have a high basal level of motility [33]. They also show a high degree of invasion through normal brain tissues [34]. Furthermore, U87MG cells show high resistance to radiation [35]. Hence, U87MG cells recapitulate many of the features of glioblastomas that make them hard to cure. New therapies are desperately needed to treat these tumors. We show in this paper that the HIV protease inhibitors nelfinavir and amprenavir can downregulate both VEGF and HIF-1α and can decrease angiogenesis in U87MG cells. We believe that the mechanism by which HIV protease inhibitors downregulate VEGF and HIF-1α involves inhibition of the PI3K pathway. This pathway has been shown to regulate HIF-1α [20,22,23]. The downregulation of VEGF by these protease inhibitors is likely multifactorial as VEGF can be regulated by the PI3K pathway through both HIF-1α dependent and HIF-1α-independent mechanisms [21,28].Wehave some evidence that these drugs act through the PI3K pathway to decrease VEGF and HIF-1α expression, although this may not be the complete story. If this is an important mechanism, this may give these drugs some specificity in targeting tumor cells. GBMs often display activation of the PI3K/Akt pathway [18,19]. This pathway should not be active in most normal tissues; therefore, in theory, its inhibition should increase the therapeutic ratio by enhancing tumor cell killing while sparing normal tissues. Consistent with this, we did not find these drugs to lead to any decrease in VEGF expression in immortalized human astrocytes (NHA). In contrast, in U87MG and U251MG cells, both of which display activation of the PI3K/Akt pathway secondary to PTEN mutation, nelfinavir decreased VEGF secretion.
These findings may have important clinical implications. There is currently great interest in identifying VEGF and HIF-1α inhibitors for use as antitumor agents. The protease inhibitors nelfinavir and amprenavir inhibit both of these targets. Although some studies suggest that inhibition of either of these targets by themselves may be sufficient to inhibit tumor growth, it is very possible that this inhibition must be combined with other modalities to be of clinical benefit. In one study, reduction of VEGF secretion in U87 cells using siRNA was not able to reduce tumor growth; however, when coupled to the antiangiogenic effect of IL-4, tumor growth was totally abolished [36]. Clinical studies that yielded positive results using the anti-VEGF monoclonal antibody bevacizumab have used it in conjunction with conventional chemotherapy [37]. For example, in a phase III randomized trial for metastatic colon cancer, patients who received standard chemotherapy and bevacizumab had improved survival compared to those who received chemotherapy and placebo [6].
In particular, the combination of anti-VEGF therapy and radiation is attractive [38,39]. One group reported that secretion of VEGF is increased by irradiation of GBM lines, which led them to speculate that this might be associated with radioresistance that could be countered using an anti-VEGF agent [40]. Several reports in the literature suggest that decreasing VEGF expression following radiation can augment the response of tumors to radiation in vivo [41–43]. Inhibition of HIF-1Ó decreases VEGF but also has the added benefit of decreasing the expression of other genes that might promote survival, such as those affecting glucose metabolism. A number of putative HIF-1α inhibitors have been identified, and many of them are currently being tested in clinical trials [44]. mTOR inhibitors such as CCI-779 have been shown to inhibit HIF-1α activity [45] and have shown efficacy in phase II clinical trials, especially in renal cell carcinoma. However, as in the case of VEGF inhibitors, HIF-1α inhibitors may prove even more useful when used in combination with conventional therapies. In support of this idea, a recent report suggests that HIF-1 blockade can promote tumor radiosensitization [46]. Previous results from our group indicate that protease inhibitors can radiosensitize cells both in vitro and in vivo [24]. The results in the current report suggest a potential mechanism by which these agents may radiosensitize tumors in vivo—by inhibition of HIF-1α/VEGF. One advantage to these drugs is that they have been in clinical use in HIV patients for over a decade, with relatively little toxicity [47]. Hence, they could be used in future clinical trials in patients with glioblastomas.
Abbreviations
- NFV
nelfinavir
- AMP
amprenavir
- DOX
doxycycline
Footnotes
This work was supported by Public Health Service grant R01 CA093638 (A.M.).
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(3):11–16. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 3.Gagner JP, Law M, Fischer I, Newcomb EW, Zagzag D. Angiogenesis in gliomas: imaging and experimental therapeutics. Brain Pathol. 2005;15:342–363. doi: 10.1111/j.1750-3639.2005.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 5.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Evans SM, Judy KD, Dunphy I, Jenkins WT, Hwang WT, Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10:8177–8184. doi: 10.1158/1078-0432.CCR-04-1081. [DOI] [PubMed] [Google Scholar]
- 8.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 10.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 11.Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 12.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 13.Dang DT, Chen F, Gardner LB, Cummins JM, Rago C, Bunz F, Kantsevoy SV, Dang LH. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 2006;66:1684–1936. doi: 10.1158/0008-5472.CAN-05-2887. [DOI] [PubMed] [Google Scholar]
- 14.Kurokawa T, Miyamoto M, Kato K, Cho Y, Kawarada Y, Hida Y, Shinohara T, Itoh T, Okushiba S, Kondo S, et al. Overexpression of hypoxia-inducible-factor 1alpha (HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer. 2003;89:1042–1047. doi: 10.1038/sj.bjc.6601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachtiary B, Schindl M, Potter R, Dreier B, Knocke TH, Hainfellner JA, Horvat R, Birner P. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234–2240. [PubMed] [Google Scholar]
- 16.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 17.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- 18.Chakravarti A, Zhai G, Suzuki Y, Sarkesh S, Black PM, Muzikansky A, Loeffler JS. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 19.Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3V-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 20.Pore N, Jiang Z, Shu H-K, Bernhard E, Kao G, Maity A. Akt activation can augment HIF-1α expression by increasing protein translation through an mTOR-independent pathway. Mol Cancer Res. 2006;4:471–479. doi: 10.1158/1541-7786.MCR-05-0234. [DOI] [PubMed] [Google Scholar]
- 21.Pore N, Liu S, Shu HK, Li B, Haas-Kogan D, Stokoe D, Milanini-Mongiat J, Pages G, O'Rourke DM, Bernhard E, et al. Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol Biol Cell. 2004;15:4841–4853. doi: 10.1091/mbc.E04-05-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxiainducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 23.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res. 2005;65:8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 25.Radu A, Neubauer V, Akagi T, Hanafusa H, Georgescu MM. PTEN induces cell cycle arrest by decreasing the level and nuclear localization of cyclin D1. Mol Cell Biol. 2003;23:6139–6149. doi: 10.1128/MCB.23.17.6139-6149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch C. In: Sen C, Packer L, editors. Methods in Enzymology: Antioxidants and Redox Cycling. Vol. 353. San Diego: Academic Press; 2002. pp. 3–31. [Google Scholar]
- 27.Koch CJ. A thin-film culturing technique allowing rapid gas-liquid equilibration (6 sec) with no toxicity to mammalian cells. Radiat Res. 1984;97:434–442. [PubMed] [Google Scholar]
- 28.Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res. 2006;66:3197–3204. doi: 10.1158/0008-5472.CAN-05-3090. [DOI] [PubMed] [Google Scholar]
- 29.Haas-Kogan D, Shalev N, Wong M, Mills G, Yount G, Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 30.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxiainducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HK. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidylinositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–4602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 33.Cattaneo M, Gentilini D, Vicentini L. Deregulated human glioma cell motility: inhibitory effect of somatostatin. Mol Cell Endocrinol. 2006;256:34–39. doi: 10.1016/j.mce.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Jung S, Ackerley C, Ivanchuk S, Mondal S, Becker L, Rutka J. Tracking the invasiveness of human astrocytoma cells by using green fluorescent protein in an organotypical brain slice model. J Neurosurg. 2001;94:80–89. doi: 10.3171/jns.2001.94.1.0080. [DOI] [PubMed] [Google Scholar]
- 35.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 36.Niola F, Evangelisti C, Campagnolo L, Massalini S, Bue MC, Mangiola A, Masotti A, Maira G, Farace MG, Ciafre SA. A plasmidencoded VEGF siRNA reduces glioblastoma angiogenesis and its combination with interleukin-4 blocks tumor growth in a xenograft mouse model. Cancer Biol Ther. 2006;5:174–179. doi: 10.4161/cbt.5.2.2317. [DOI] [PubMed] [Google Scholar]
- 37.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 38.O'Reilly MS. Radiation combined with antiangiogenic and antivascular agents. Semin Radiat Oncol. 2006;16:45–50. doi: 10.1016/j.semradonc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Wachsberger P, Burd R, Dicker AP. Improving tumor response to radiotherapy by targeting angiogenesis signaling pathways. Hematol Oncol Clin North Am. 2004;18:1039–1057. doi: 10.1016/j.hoc.2004.06.007. (viii) [DOI] [PubMed] [Google Scholar]
- 40.Hovinga KE, Stalpers LJ, van Bree C, Donker M, Verhoeff JJ, Rodermond HM, Bosch DA, van Furth WR. Radiation-enhanced vascular endothelial growth factor (VEGF) secretion in glioblastoma multiforme cell lines—a clue to radioresistance? J Neuro-Oncol. 2005;74:99–103. doi: 10.1007/s11060-004-4204-7. [DOI] [PubMed] [Google Scholar]
- 41.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 42.Hess C, Vuong V, Hegyi I, Riesterer O, Wood J, Fabbro D, Glanzmann C, Bodis S, Pruschy M. Effect of VEGF receptor inhibitor PTK787/ZK222584 [correction of ZK222548] combined with ionizing radiation on endothelial cells and tumour growth. Br J Cancer. 2001;85:2010–2016. doi: 10.1054/bjoc.2001.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zips D, Eicheler W, Geyer P, Hessel F, Dorfler A, Thames HD, Haberey M, Baumann M. Enhanced susceptibility of irradiated tumor vessels to vascular endothelial growth factor receptor tyrosine kinase inhibition. Cancer Res. 2005;65:5374–5379. doi: 10.1158/0008-5472.CAN-04-3379. [DOI] [PubMed] [Google Scholar]
- 44.Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 45.Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 46.Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Powderly WG. Long-term exposure to lifelong therapies. J Acquir Immune Defic Syndr. 2002;29(1):S28–S40. doi: 10.1097/00126334-200202011-00005. [DOI] [PubMed] [Google Scholar]