Abstract
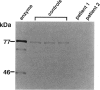
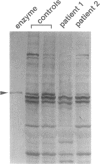
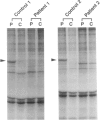
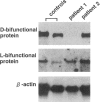
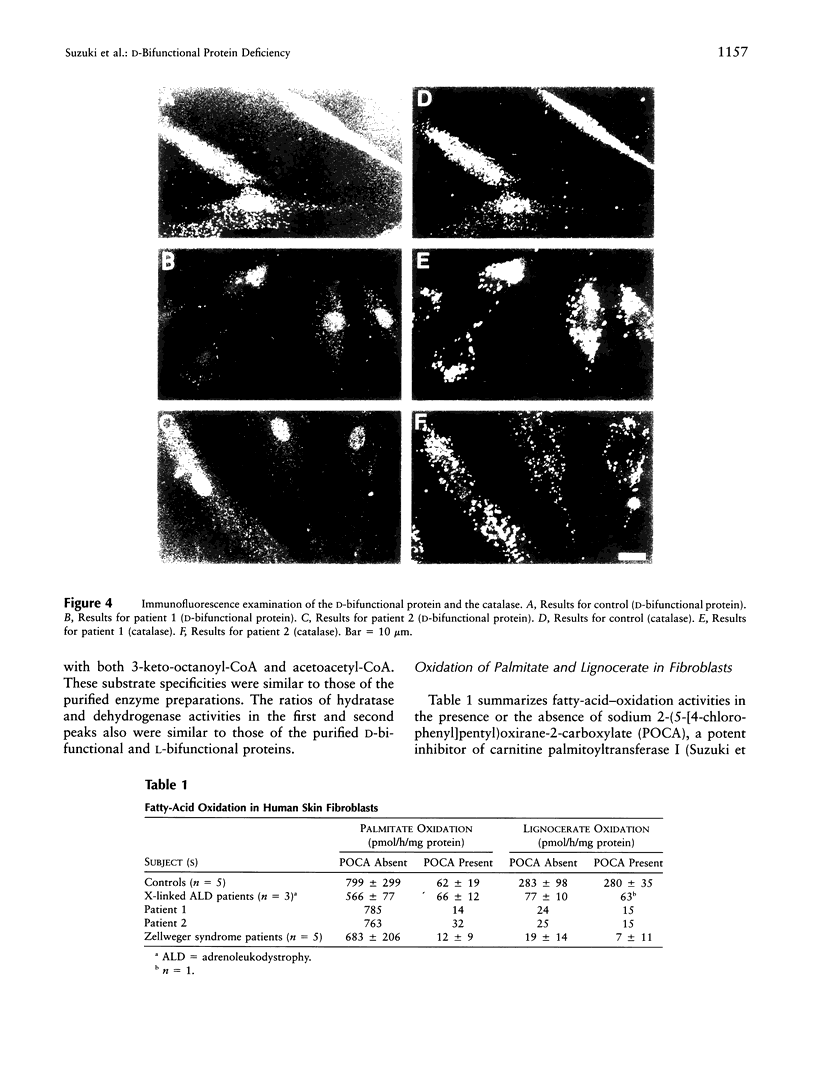
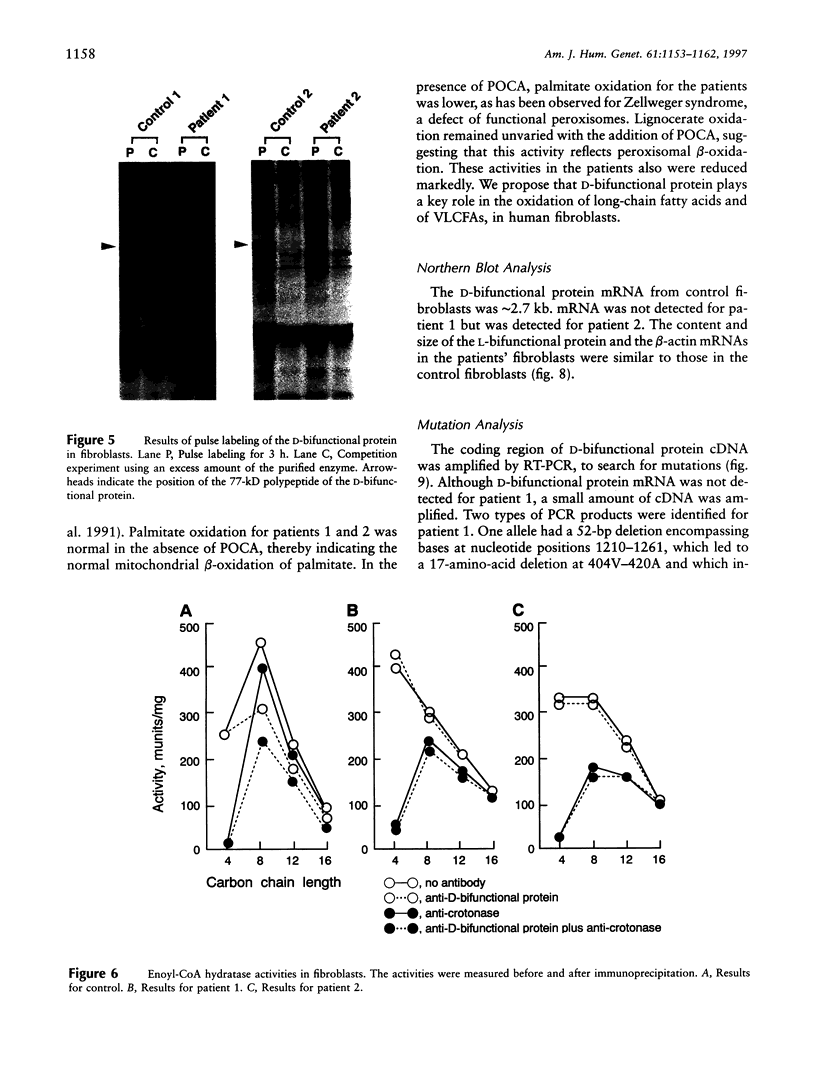
Peroxisomal beta-oxidation proceeds from enoyl-CoA through D-3-hydroxyacyl-CoA to 3-ketoacyl-CoA by the D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxy-acyl-CoA dehydrogenase bifunctional protein (d-bifunctional protein), and the oxidation of bile-acid precursors also has been suggested as being catalyzed by the d-bifunctional protein. Because of the important roles of this protein, we reinvestigated two Japanese patients previously diagnosed as having enoyl-CoA hydratase/L-3-hydroxyacyl-CoA dehydrogenase bifunctional protein (L-bifunctional protein) deficiency, in complementation studies. We found that both the protein and the enzyme activity of the d-bifunctional protein were hardly detectable in these patients but that the active L-bifunctional protein was present. The mRNA level in patient 1 was very low, and, for patient 2, mRNA was of a smaller size. Sequencing analysis of the cDNA revealed a 52-bp deletion in patient 1 and a 237-bp deletion in patient 2. This seems to be the first report of D-bifunctional protein deficiency. Patients previously diagnosed as cases of L-bifunctional protein deficiency probably should be reexamined for a possible d-bifunctional protein deficiency.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamski J., Normand T., Leenders F., Monté D., Begue A., Stéhelin D., Jungblut P. W., de Launoit Y. Molecular cloning of a novel widely expressed human 80 kDa 17 beta-hydroxysteroid dehydrogenase IV. Biochem J. 1995 Oct 15;311(Pt 2):437–443. doi: 10.1042/bj3110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clayton P. T., Lake B. D., Hjelm M., Stephenson J. B., Besley G. T., Wanders R. J., Schram A. W., Tager J. M., Schutgens R. B., Lawson A. M. Bile acid analyses in "pseudo-Zellweger" syndrome; clues to the defect in peroxisomal beta-oxidation. J Inherit Metab Dis. 1988;11 (Suppl 2):165–168. doi: 10.1007/BF01804226. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Osumi T., Hashimoto T., Ui N. Properties of mitochondria and peroxisomal enoyl-CoA hydratases from rat liver. J Biochem. 1980 Oct;88(4):1059–1070. doi: 10.1093/oxfordjournals.jbchem.a133057. [DOI] [PubMed] [Google Scholar]
- Hashimoto T. Individual peroxisomal beta-oxidation enzymes. Ann N Y Acad Sci. 1982;386:5–12. doi: 10.1111/j.1749-6632.1982.tb21403.x. [DOI] [PubMed] [Google Scholar]
- Jiang L. L., Kobayashi A., Matsuura H., Fukushima H., Hashimoto T. Purification and properties of human D-3-hydroxyacyl-CoA dehydratase: medium-chain enoyl-CoA hydratase is D-3-hydroxyacyl-CoA dehydratase. J Biochem. 1996 Sep;120(3):624–632. doi: 10.1093/oxfordjournals.jbchem.a021458. [DOI] [PubMed] [Google Scholar]
- Jiang L. L., Kurosawa T., Sato M., Suzuki Y., Hashimoto T. Physiological role of D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J Biochem. 1997 Mar;121(3):506–513. doi: 10.1093/oxfordjournals.jbchem.a021615. [DOI] [PubMed] [Google Scholar]
- Jiang L. L., Miyazawa S., Hashimoto T. Purification and properties of rat D-3-hydroxyacyl-CoA dehydratase: D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J Biochem. 1996 Sep;120(3):633–641. doi: 10.1093/oxfordjournals.jbchem.a021459. [DOI] [PubMed] [Google Scholar]
- Jiang L. L., Miyazawa S., Souri M., Hashimoto T. Structure of D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J Biochem. 1997 Feb;121(2):364–369. doi: 10.1093/oxfordjournals.jbchem.a021596. [DOI] [PubMed] [Google Scholar]
- Kobayashi A., Jiang L. L., Hashimoto T. Two mitochondrial 3-hydroxyacyl-CoA dehydrogenases in bovine liver. J Biochem. 1996 Apr;119(4):775–782. doi: 10.1093/oxfordjournals.jbchem.a021307. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Dommes V., Schulz H. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res. 1995;34(4):267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Osumi T., Hashimoto T. The presence of a new 3-oxoacyl-CoA thiolase in rat liver peroxisomes. Eur J Biochem. 1980 Feb;103(3):589–596. doi: 10.1111/j.1432-1033.1980.tb05984.x. [DOI] [PubMed] [Google Scholar]
- Nakada Y., Hyakuna N., Suzuki Y., Shimozawa N., Takaesu E., Ikema R., Hirayama K. A case of pseudo-Zellweger syndrome with a possible bifunctional enzyme deficiency but detectable enzyme protein. Comparison of two cases of Zellweger syndrome. Brain Dev. 1993 Nov-Dec;15(6):453–456. doi: 10.1016/0387-7604(93)90087-o. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natowicz M. R., Evans J. E., Kelley R. I., Moser A. B., Watkins P. A., Moser H. W. Urinary bile acids and peroxisomal bifunctional enzyme deficiency. Am J Med Genet. 1996 May 17;63(2):356–362. doi: 10.1002/(SICI)1096-8628(19960517)63:2<356::AID-AJMG6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Osumi T., Hashimoto T., Ui N. Purification and properties of acyl-CoA oxidase from rat liver. J Biochem. 1980 Jun;87(6):1735–1746. doi: 10.1093/oxfordjournals.jbchem.a132918. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I. Peroxisomal oxidation of the steroid side chain in bile acid formation. Biochimie. 1993;75(3-4):159–165. doi: 10.1016/0300-9084(93)90073-2. [DOI] [PubMed] [Google Scholar]
- Poll-The B. T., Roels F., Ogier H., Scotto J., Vamecq J., Schutgens R. B., Wanders R. J., van Roermund C. W., van Wijland M. J., Schram A. W. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am J Hum Genet. 1988 Mar;42(3):422–434. [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Mannaerts G. P. Peroxisomal lipid metabolism. Annu Rev Nutr. 1994;14:343–370. doi: 10.1146/annurev.nu.14.070194.002015. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram A. W., Goldfischer S., van Roermund C. W., Brouwer-Kelder E. M., Collins J., Hashimoto T., Heymans H. S., van den Bosch H., Schutgens R. B., Tager J. M. Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2494–2496. doi: 10.1073/pnas.84.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Usher S., Johnson D., Poulos A. A comparative study of straight chain and branched chain fatty acid oxidation in skin fibroblasts from patients with peroxisomal disorders. J Lipid Res. 1990 Feb;31(2):217–225. [PubMed] [Google Scholar]
- Suzuki Y., Shimozawa N., Yajima S., Tomatsu S., Kondo N., Nakada Y., Akaboshi S., Lai M., Tanabe Y., Hashimoto T. Novel subtype of peroxisomal acyl-CoA oxidase deficiency and bifunctional enzyme deficiency with detectable enzyme protein: identification by means of complementation analysis. Am J Hum Genet. 1994 Jan;54(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Shimozawa N., Yajima S., Yamaguchi S., Orii T., Hashimoto T. Effects of sodium 2-[5-(4-chlorophenyl)pentyl]-oxirane-2-carboxylate (POCA) on fatty acid oxidation in fibroblasts from patients with peroxisomal diseases. Biochem Pharmacol. 1991 Feb 1;41(3):453–456. doi: 10.1016/0006-2952(91)90544-f. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Huang S., Eyssen H. J., Mannaerts G. P. The deficient degradation of synthetic 2- and 3-methyl-branched fatty acids in fibroblasts from patients with peroxisomal disorders. J Inherit Metab Dis. 1993;16(2):381–391. doi: 10.1007/BF00710285. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., Brul S., Schutgens R. B., Tager J. M. Bifunctional enzyme deficiency: identification of a new type of peroxisomal disorder in a patient with an impairment in peroxisomal beta-oxidation of unknown aetiology by means of complementation analysis. J Inherit Metab Dis. 1992;15(3):385–388. doi: 10.1007/BF02435983. [DOI] [PubMed] [Google Scholar]
- Wanders R. J., van Roermund C. W., Schelen A., Schutgens R. B., Tager J. M., Stephenson J. B., Clayton P. T. A bifunctional protein with deficient enzymic activity: identification of a new peroxisomal disorder using novel methods to measure the peroxisomal beta-oxidation enzyme activities. J Inherit Metab Dis. 1990;13(3):375–379. doi: 10.1007/BF01799399. [DOI] [PubMed] [Google Scholar]
- Watkins P. A., Chen W. W., Harris C. J., Hoefler G., Hoefler S., Blake D. C., Jr, Balfe A., Kelley R. I., Moser A. B., Beard M. E. Peroxisomal bifunctional enzyme deficiency. J Clin Invest. 1989 Mar;83(3):771–777. doi: 10.1172/JCI113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins P. A., McGuinness M. C., Raymond G. V., Hicks B. A., Sisk J. M., Moser A. B., Moser H. W. Distinction between peroxisomal bifunctional enzyme and acyl-CoA oxidase deficiencies. Ann Neurol. 1995 Sep;38(3):472–477. doi: 10.1002/ana.410380322. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Orii T., Sakura N., Miyazawa S., Hashimoto T. Defect in biosynthesis of mitochondrial acetoacetyl-coenzyme A thiolase in cultured fibroblasts from a boy with 3-ketothiolase deficiency. J Clin Invest. 1988 Mar;81(3):813–817. doi: 10.1172/JCI113388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brink H. J., Poll-The B. T., Saudubray J. M., Wanders R. J., Jakobs C. Pristanic acid does not accumulate in peroxisomal acyl-CoA oxidase deficiency: evidence for a distinct peroxisomal pristanyl-CoA oxidase. J Inherit Metab Dis. 1991;14(5):681–684. doi: 10.1007/BF01799934. [DOI] [PubMed] [Google Scholar]
- ten Brink H. J., Wanders R. J., Stellaard F., Schutgens R. B., Jakobs C. Pristanic acid and phytanic acid in plasma from patients with a single peroxisomal enzyme deficiency. J Inherit Metab Dis. 1991;14(3):345–348. doi: 10.1007/BF01811699. [DOI] [PubMed] [Google Scholar]