Abstract
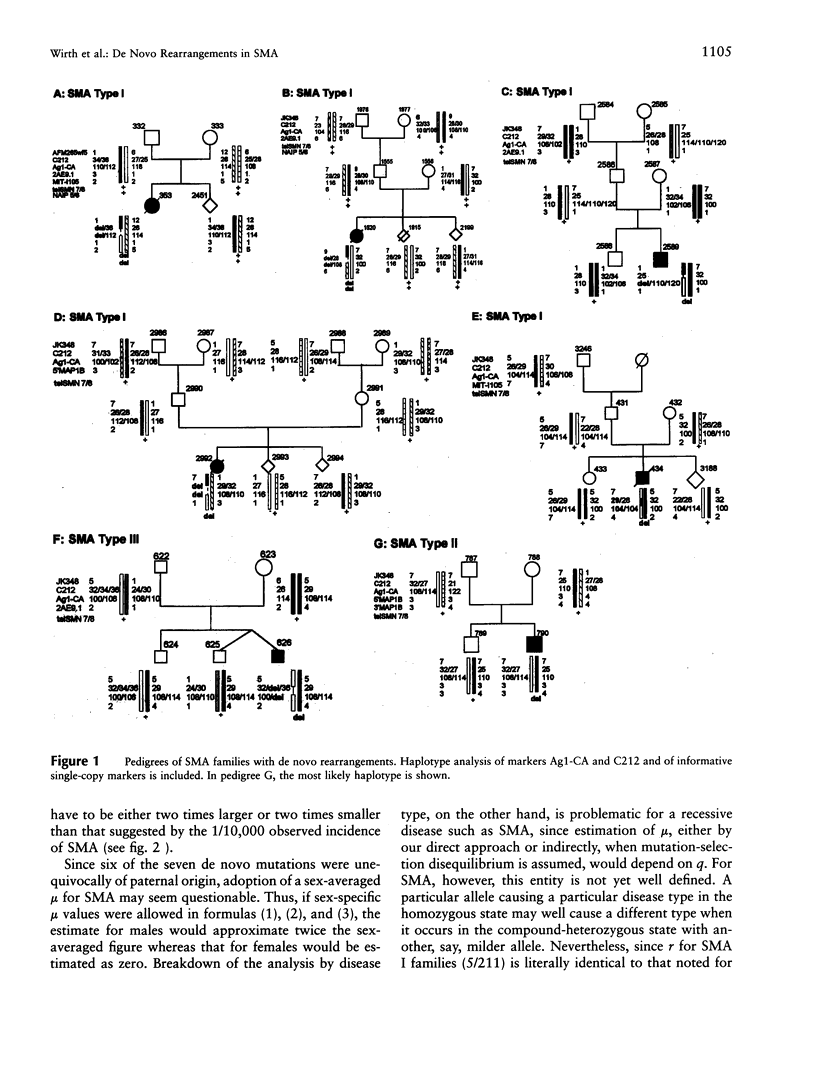
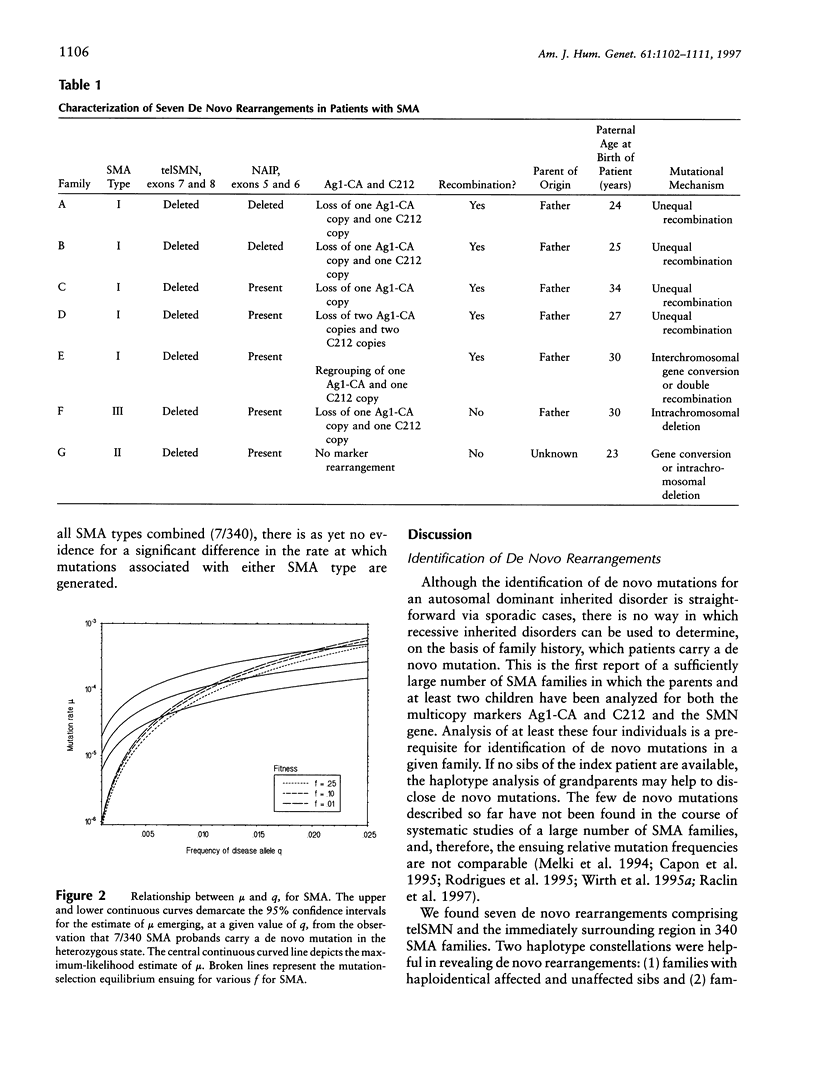
Spinal muscular atrophy (SMA) is a relatively common autosomal recessive neuromuscular disorder. We have identified de novo rearrangements in 7 (approximately 2%) index patients from 340 informative SMA families. In each, the rearrangements resulted in the absence of the telomeric copy of the survival motor neuron (SMN) gene (telSMN), in two cases accompanied by the loss of the neuronal apoptosis-inhibitory protein gene . Haplotype analysis revealed unequal recombination in four cases, with loss of markers Ag1-CA and C212, which are near the 5' ends of the SMN genes. In one case, an interchromosomal rearrangement involving both the SMN genes and a regrouping of Ag1-CA and C212 alleles must have occurred, suggesting either interchromosomal gene conversion or double recombination. In two cases, no such rearrangement was observed, but loss of telSMN plus Ag1-CA and C212 alleles in one case suggested intrachromosomal deletion or gene conversion. In six of the seven cases, the de novo rearrangement had occurred during paternal meiosis. Direct detection of de novo SMA mutations by molecular genetic means has allowed us to estimate for the first time the mutation rate for a recessive disorder in humans. The sex-averaged rate of 1.1 x 10(-4), arrived at in a proband-based approach, compares well with the rate of 0.9 x 10(-4) expected under a mutation-selection equilibrium for SMA. These findings have important implications for genetic counseling and prenatal diagnosis in that they emphasize the relevance of indirect genotype analysis in combination with direct SMN-gene deletion testing in SMA families.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair I. P., Nash J., Gordon M. J., Nicholson G. A. Prevalence and origin of de novo duplications in Charcot-Marie-Tooth disease type 1A: first report of a de novo duplication with a maternal origin. Am J Hum Genet. 1996 Mar;58(3):472–476. [PMC free article] [PubMed] [Google Scholar]
- Brahe C., Clermont O., Zappata S., Tiziano F., Melki J., Neri G. Frameshift mutation in the survival motor neuron gene in a severe case of SMA type I. Hum Mol Genet. 1996 Dec;5(12):1971–1976. doi: 10.1093/hmg/5.12.1971. [DOI] [PubMed] [Google Scholar]
- Brzustowicz L. M., Lehner T., Castilla L. H., Penchaszadeh G. K., Wilhelmsen K. C., Daniels R., Davies K. E., Leppert M., Ziter F., Wood D. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2-13.3. Nature. 1990 Apr 5;344(6266):540–541. doi: 10.1038/344540a0. [DOI] [PubMed] [Google Scholar]
- Burghes A. H., Ingraham S. E., McLean M., Thompson T. G., McPherson J. D., Kote-Jarai Z., Carpten J. D., DiDonato C. J., Ikeda J. E., Surh L. A multicopy dinucleotide marker that maps close to the spinal muscular atrophy gene. Genomics. 1994 May 15;21(2):394–402. doi: 10.1006/geno.1994.1282. [DOI] [PubMed] [Google Scholar]
- Burghes A. H. When is a deletion not a deletion? When it is converted. Am J Hum Genet. 1997 Jul;61(1):9–15. doi: 10.1086/513913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussaglia E., Clermont O., Tizzano E., Lefebvre S., Bürglen L., Cruaud C., Urtizberea J. A., Colomer J., Munnich A., Baiget M. A frame-shift deletion in the survival motor neuron gene in Spanish spinal muscular atrophy patients. Nat Genet. 1995 Nov;11(3):335–337. doi: 10.1038/ng1195-335. [DOI] [PubMed] [Google Scholar]
- Bürglen L., Lefebvre S., Clermont O., Burlet P., Viollet L., Cruaud C., Munnich A., Melki J. Structure and organization of the human survival motor neurone (SMN) gene. Genomics. 1996 Mar 15;32(3):479–482. doi: 10.1006/geno.1996.0147. [DOI] [PubMed] [Google Scholar]
- Bürglen L., Seroz T., Miniou P., Lefebvre S., Burlet P., Munnich A., Pequignot E. V., Egly J. M., Melki J. The gene encoding p44, a subunit of the transcription factor TFIIH, is involved in large-scale deletions associated with Werdnig-Hoffmann disease. Am J Hum Genet. 1997 Jan;60(1):72–79. [PMC free article] [PubMed] [Google Scholar]
- Campbell L., Potter A., Ignatius J., Dubowitz V., Davies K. Genomic variation and gene conversion in spinal muscular atrophy: implications for disease process and clinical phenotype. Am J Hum Genet. 1997 Jul;61(1):40–50. doi: 10.1086/513886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon F., Levato C., Bussaglia E., Lo Cicero S., Tizzano E. F., Baiget M., Silani V., Pizzuti A., Novelli G., Dallapiccola B. Deletion analysis of the simple tandem repeat loci physically linked to the spinal muscular atrophy locus. Hum Mutat. 1996;7(3):198–201. doi: 10.1002/(SICI)1098-1004(1996)7:3<198::AID-HUMU3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Capon F., Lo Cicero S., Levato C., Novelli G., Dallapiccola B. De novo deletions of the 5q13 region and prenatal diagnosis of spinal muscular atrophy. Prenat Diagn. 1995 Jan;15(1):93–94. doi: 10.1002/pd.1970150121. [DOI] [PubMed] [Google Scholar]
- Chandley A. C. Asymmetry in chromosome pairing: a major factor in de novo mutation and the production of genetic disease in man. J Med Genet. 1989 Sep;26(9):546–552. doi: 10.1136/jmg.26.9.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobben J. M., van der Steege G., Grootscholten P., de Visser M., Scheffer H., Buys C. H. Deletions of the survival motor neuron gene in unaffected siblings of patients with spinal muscular atrophy. Am J Hum Genet. 1995 Oct;57(4):805–808. [PMC free article] [PubMed] [Google Scholar]
- Cremonesi L., Cainarca S., Rossi A., Padoan R., Ferrari M. Detection of a de novo R1066H mutation in an Italian patient affected by cystic fibrosis. Hum Genet. 1996 Jul;98(1):119–121. doi: 10.1007/s004390050171. [DOI] [PubMed] [Google Scholar]
- DiDonato C. J., Ingraham S. E., Mendell J. R., Prior T. W., Lenard S., Moxley R. T., 3rd, Florence J., Burghes A. H. Deletion and conversion in spinal muscular atrophy patients: is there a relationship to severity? Ann Neurol. 1997 Feb;41(2):230–237. doi: 10.1002/ana.410410214. [DOI] [PubMed] [Google Scholar]
- DiDonato C. J., Morgan K., Carpten J. D., Fuerst P., Ingraham S. E., Prescott G., McPherson J. D., Wirth B., Zerres K., Hurko O. Association between Ag1-CA alleles and severity of autosomal recessive proximal spinal muscular atrophy. Am J Hum Genet. 1994 Dec;55(6):1218–1229. [PMC free article] [PubMed] [Google Scholar]
- Gabriel S. E., Brigman K. N., Koller B. H., Boucher R. C., Stutts M. J. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994 Oct 7;266(5182):107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- Gilliam T. C., Brzustowicz L. M., Castilla L. H., Lehner T., Penchaszadeh G. K., Daniels R. J., Byth B. C., Knowles J., Hislop J. E., Shapira Y. Genetic homogeneity between acute and chronic forms of spinal muscular atrophy. Nature. 1990 Jun 28;345(6278):823–825. doi: 10.1038/345823a0. [DOI] [PubMed] [Google Scholar]
- Hahnen E. T., Wirth B. Frequent DNA variant in exon 2a of the survival motor neuron gene (SMN): a further possibility for distinguishing the two copies of the gene. Hum Genet. 1996 Jul;98(1):122–123. doi: 10.1007/s004390050172. [DOI] [PubMed] [Google Scholar]
- Hahnen E., Forkert R., Marke C., Rudnik-Schöneborn S., Schönling J., Zerres K., Wirth B. Molecular analysis of candidate genes on chromosome 5q13 in autosomal recessive spinal muscular atrophy: evidence of homozygous deletions of the SMN gene in unaffected individuals. Hum Mol Genet. 1995 Oct;4(10):1927–1933. doi: 10.1093/hmg/4.10.1927. [DOI] [PubMed] [Google Scholar]
- Hahnen E., Schönling J., Rudnik-Schöneborn S., Zerres K., Wirth B. Hybrid survival motor neuron genes in patients with autosomal recessive spinal muscular atrophy: new insights into molecular mechanisms responsible for the disease. Am J Hum Genet. 1996 Nov;59(5):1057–1065. [PMC free article] [PubMed] [Google Scholar]
- LeGuern E., Gouider R., Ravisé N., Lopes J., Tardieu S., Gugenheim M., Abbas N., Bouche P., Agid Y., Brice A. A de novo case of hereditary neuropathy with liability to pressure palsies (HNPP) of maternal origin: a new mechanism for deletion in 17p11.2? Hum Mol Genet. 1996 Jan;5(1):103–106. doi: 10.1093/hmg/5.1.103. [DOI] [PubMed] [Google Scholar]
- Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995 Jan 13;80(1):155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- McAndrew P. E., Parsons D. W., Simard L. R., Rochette C., Ray P. N., Mendell J. R., Prior T. W., Burghes A. H. Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am J Hum Genet. 1997 Jun;60(6):1411–1422. doi: 10.1086/515465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki J., Abdelhak S., Sheth P., Bachelot M. F., Burlet P., Marcadet A., Aicardi J., Barois A., Carriere J. P., Fardeau M. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature. 1990 Apr 19;344(6268):767–768. doi: 10.1038/344767a0. [DOI] [PubMed] [Google Scholar]
- Melki J., Lefebvre S., Burglen L., Burlet P., Clermont O., Millasseau P., Reboullet S., Bénichou B., Zeviani M., Le Paslier D. De novo and inherited deletions of the 5q13 region in spinal muscular atrophies. Science. 1994 Jun 3;264(5164):1474–1477. doi: 10.1126/science.7910982. [DOI] [PubMed] [Google Scholar]
- Melki J., Sheth P., Abdelhak S., Burlet P., Bachelot M. F., Lathrop M. G., Frezal J., Munnich A. Mapping of acute (type I) spinal muscular atrophy to chromosome 5q12-q14. The French Spinal Muscular Atrophy Investigators. Lancet. 1990 Aug 4;336(8710):271–273. doi: 10.1016/0140-6736(90)91803-i. [DOI] [PubMed] [Google Scholar]
- Moloney D. M., Slaney S. F., Oldridge M., Wall S. A., Sahlin P., Stenman G., Wilkie A. O. Exclusive paternal origin of new mutations in Apert syndrome. Nat Genet. 1996 May;13(1):48–53. doi: 10.1038/ng0596-48. [DOI] [PubMed] [Google Scholar]
- Palau F., Löfgren A., De Jonghe P., Bort S., Nelis E., Sevilla T., Martin J. J., Vilchez J., Prieto F., Van Broeckhoven C. Origin of the de novo duplication in Charcot-Marie-Tooth disease type 1A: unequal nonsister chromatid exchange during spermatogenesis. Hum Mol Genet. 1993 Dec;2(12):2031–2035. doi: 10.1093/hmg/2.12.2031. [DOI] [PubMed] [Google Scholar]
- Parsons D. W., McAndrew P. E., Monani U. R., Mendell J. R., Burghes A. H., Prior T. W. An 11 base pair duplication in exon 6 of the SMN gene produces a type I spinal muscular atrophy (SMA) phenotype: further evidence for SMN as the primary SMA-determining gene. Hum Mol Genet. 1996 Nov;5(11):1727–1732. doi: 10.1093/hmg/5.11.1727. [DOI] [PubMed] [Google Scholar]
- Raclin V., Veber P. S., Bürglen L., Munnich A., Melki J. De novo deletions in spinal muscular atrophy: implications for genetic counselling. J Med Genet. 1997 Jan;34(1):86–87. doi: 10.1136/jmg.34.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues N. R., Owen N., Talbot K., Ignatius J., Dubowitz V., Davies K. E. Deletions in the survival motor neuron gene on 5q13 in autosomal recessive spinal muscular atrophy. Hum Mol Genet. 1995 Apr;4(4):631–634. doi: 10.1093/hmg/4.4.631. [DOI] [PubMed] [Google Scholar]
- Roy N., Mahadevan M. S., McLean M., Shutler G., Yaraghi Z., Farahani R., Baird S., Besner-Johnston A., Lefebvre C., Kang X. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995 Jan 13;80(1):167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- Rudnik-Schöneborn S., Zerres K., Hahnen E., Meng G., Voit T., Hanefeld F., Wirth B. Apparent autosomal recessive inheritance in families with proximal spinal muscular atrophy affecting individuals in two generations. Am J Hum Genet. 1996 Nov;59(5):1163–1165. [PMC free article] [PubMed] [Google Scholar]
- Talbot K., Ponting C. P., Theodosiou A. M., Rodrigues N. R., Surtees R., Mountford R., Davies K. E. Missense mutation clustering in the survival motor neuron gene: a role for a conserved tyrosine and glycine rich region of the protein in RNA metabolism? Hum Mol Genet. 1997 Mar;6(3):497–500. doi: 10.1093/hmg/6.3.497. [DOI] [PubMed] [Google Scholar]
- Wang C. H., Xu J., Carter T. A., Ross B. M., Dominski M. K., Bellcross C. A., Penchaszadeh G. K., Munsat T. L., Gilliam T. C. Characterization of survival motor neuron (SMNT) gene deletions in asymptomatic carriers of spinal muscular atrophy. Hum Mol Genet. 1996 Mar;5(3):359–365. doi: 10.1093/hmg/5.3.359. [DOI] [PubMed] [Google Scholar]
- White M. B., Leppert M., Nielsen D., Zielenski J., Gerrard B., Stewart C., Dean M. A de novo cystic fibrosis mutation: CGA (Arg) to TGA (stop) at codon 851 of the CFTR gene. Genomics. 1991 Nov;11(3):778–779. doi: 10.1016/0888-7543(91)90092-s. [DOI] [PubMed] [Google Scholar]
- Wirth B., Hahnen E., Morgan K., DiDonato C. J., Dadze A., Rudnik-Schöneborn S., Simard L. R., Zerres K., Burghes A. H. Allelic association and deletions in autosomal recessive proximal spinal muscular atrophy: association of marker genotype with disease severity and candidate cDNAs. Hum Mol Genet. 1995 Aug;4(8):1273–1284. doi: 10.1093/hmg/4.8.1273. [DOI] [PubMed] [Google Scholar]
- Wirth B., Pick E., Leutner A., Dadze A., Voosen B., Knapp M., Piechaczek-Wappenschmidt B., Rudnik-Schöneborn S., Schönling J., Cox S. Large linkage analysis in 100 families with autosomal recessive spinal muscular atrophy (SMA) and 11 CEPH families using 15 polymorphic loci in the region 5q11.2-q13.3. Genomics. 1994 Mar 1;20(1):84–93. doi: 10.1006/geno.1994.1130. [DOI] [PubMed] [Google Scholar]
- Wirth B., Rudnik-Schöneborn S., Hahnen E., Röhrig D., Zerres K. Prenatal prediction in families with autosomal recessive proximal spinal muscular atrophy (5q11.2-q13.3): molecular genetics and clinical experience in 109 cases. Prenat Diagn. 1995 May;15(5):407–417. doi: 10.1002/pd.1970150503. [DOI] [PubMed] [Google Scholar]
- Zerres K., Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol. 1995 May;52(5):518–523. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- van der Steege G., Grootscholten P. M., Cobben J. M., Zappata S., Scheffer H., den Dunnen J. T., van Ommen G. J., Brahe C., Buys C. H. Apparent gene conversions involving the SMN gene in the region of the spinal muscular atrophy locus on chromosome 5. Am J Hum Genet. 1996 Oct;59(4):834–838. [PMC free article] [PubMed] [Google Scholar]


