Abstract
The expression of telomerase activity and the in situ localization of the human telomerase RNA component (hTR) in melanocytic skin lesions was evaluated in specimens from sixty-three patients. Specimens of melanocytic nevi, primary melanomas and subcutaneous metastases of melanoma were obtained from fifty-eight patients, whereas metastasized lymph nodes were obtained from five patients. Telomerase activity was determined in these specimens by using a Polymerase Chain Reaction-based assay (TRAP). High relative mean telomerase activity levels were detected in metastatic melanoma (subcutaneous metastasess = 54.5, lymph node metastasess = 56.5). Much lower levels were detected in primary melanomas, which increased with advancing levels of tumor cell penetration (Clark II = 0.02, Clark III = 1.1, and Clark IV = 1.9). Twenty-six formalin-fixed, paraffin-embedded melanocytic lesions were sectioned and analyzed for telomerase RNA with a radioactive in situ hybridization assay. In situ hybridization studies with a probe to the template RNA component of telomerase confirmed that expression was almost exclusively confined to tumor cells and not infiltrating lymphocytes. These results indicate that levels of telomerase activity and telomerase RNA in melanocytic lesions correlate well with clinical stage and could potentially assist in the diagnosis of borderline lesions.
Keywords: skin cancer, immortality, telomeres, metastases, prognosis
Introduction
Telomeres, the chromosomal end regions composed of tandem repeats of the sequence TTAGGG in vertebrates [1] are believed to be important in stabilizing the chromosomes during replication [2] Because DNA polymerase is unable to completely replicate telomeric sequences [3,4] it has been proposed that progressive loss of telomere length during cell division could act as a mechanism that limits the replicative capacity of normal somatic cells [5,6] Telomerase is a ribonucleoprotein enzyme that can use its RNA [7] component as a template and the telomerase catalytic protein reverse transcriptase [8–10] for the addition of telomeric repeats to the 3′ ends of the chromosomes. Thus, telomerase is thought to partially or completely compensate for the progressive telomere erosion that would occur in its absence. Because most normal somatic cells in vertebrates do not express telomerase, it is thought that their telomeres progressively shorten, undergo genomic instability, and cease proliferating once a critical length is reached [11]. It has been suggested that upregulation or re-expression of telomerase may result in chromosomal stability leading to continuous cell proliferation [6].
Cancer is generally believed to be caused by accumulation of multiple somatic mutations that subvert the normal growth control mechanisms [12]. It has been suggested that in most cancers cellular immortality is among the changes required for sustained long-term growth [6,13,14]. Telomerase activity has been detected in 85% of tumors representing 15 cancer types, whereas most benign tumors and somatic tissues have very low levels or lack detectable telomerase activity [15,16] Furthermore, in special metastatic tumor types such as neuroblastoma stage IVs, in which there is a high rate of spontaneous regression, telomerase activity is very low or not detectable, and telomere lengths are considerably shorter than adjacent tissue [17]. Thus, although telomerase is not necessarily required for the initiation of a tumor, it is likely to be important in the sustained growth of the tumor.
The diagnosis of melanoma can be made clinically in 80% to 90% of cases [18], whereas the remainder require histological examination for diagnosis. However, a certain degree of ambiguity and thus indecision remains in the histological diagnosis of melanoma [19] To accurately pinpoint tumor cells and improve diagnosis, pathologists often require the use of neoplastic markers. Telomerase has been touted as such a marker. It has been shown previously that the human telomerase template RNA (hTR) can be used in situ to distinguish between cancer cells and normal cells in humans [20,21] and that levels of telomerase activity correlate well with the degree of hTR. Thus, in situ techniques applied to tissue samples can now be used to localize the presence of telomerase in melanoma cells thereby, potentially aiding in diagnosis.
Traditionally, once the diagnosis is confirmed, tumors are staged based on the clinical and/or radiographic evidence of spread through the body. Clinical staging has been critical to determining treatment and prognosis. If there is no indication that melanoma has spread beyond the skin (clinical stage I and II), treatment decisions are based on the anatomical level of invasion [22,23] of tumor cells into the skin. A number of histological parameters have been considered in an attempt to identify the greatest predictive value for the development of metastases [24–26] and overall survival. Tumor thickness has consistently been shown to have the most predictive value [27,28]. Two classification schemes are used by pathologists, including the Breslow thickness (penetration depth measured in millimeters) and the Clark classification (five levels of penetration based on anatomic location of tumor cells in the skin) [22,23,27,28] Because telomerase activity levels and RNA levels correlate well with degree of malignancy [20,21] it is possible that determination of telomerase RNA expression or telomerase activity levels in primary melanomas could help to determine more precisely the aggressive nature of individual tumors, thus enabling more appropriate therapies and improved prognosis.
In our present study, we analyzed and compared the levels of telomerase activity with a variety of clinical and histological parameters of a large group of cutaneous melanocytic conditions, including compound or junctional melanocytic nevi, malignant primary melanomas, subcutaneous metastasis of melanoma, and lymph nodes containing metastatic melanoma. We also used a telomerase RNA in situ hybridization assay to determine the pattern of expression of hTR on formalin-fixed paraffin-embedded melanocytic specimens.
Materials and Methods
Tissue Acquisition
All tissue samples were collected at the Istituto Dermatopatico dell'Immacolata, Rome, Italy. Specimens of melanocytic nevi, primary melanomas, and subcutaneous metastases of melanoma were obtained under local anesthesia from a total of fifty-eight patients (with informed consent) who underwent surgical removal of the lesion for histological examination. Only relatively large (> 1 cm) primary lesions were used for this study. Metastasized lymph nodes were obtained from five patients who underwent therapeutic regional lymph node dissection.
Specimens were received fresh in the surgical pathology room. Those in which a reasonably obvious diagnosis of benign or malignant nature was not possible were submitted for standard histological evaluations. Obvious subcutaneous metastases and grossly involved lymph nodes were bisected, half of which were submitted for routine histological evaluation and half stored for telomerase assays. Primary lesions were serially sectioned, and at least three sections displaying maximal thickness, as judged by gross inspection, were submitted for routine histological examination. One section from the periphery of the pigmented lesion was taken for in situ telomerase RNA analysis. Subcutaneous and lateral portions of grossly uninvolved skin were then removed with a scalpel so that only the clear-cut lesion and the overlaying epidermis remained. Samples of normal skin adjacent to primary lesions were also collected and tested for telomerase activity.
Tissue samples were rapidly frozen and stored at -80°C until they were tested for telomerase activity. The entire procedure from the time of surgery to the freezing step was performed under sterile conditions and always completed within 30 minutes.
Histopathologic Evaluation
Sections from each paraffin block were cut at 3-µm thickness and stained with hematoxylin and eosin. The diagnosis of melanoma or melanocytic nevus was established according to the standard accepted criteria. Breslow thickness was measured with a Leitz micrometer eyepiece (E. Leitz, Ruckleigh, NJ). Mitotic rate was rated as high (6 mitotic figures per mm2) or low (fewer than 6 mitotic figures per mm2). Melanoma patients were staged according to the American Joint Commission on Cancer (AJCC).
Preparation of Tissue Extracts
Tissue extracts were prepared as previously described [29] with the following modifications. Frozen specimens containing dissected samples were thawed and subsequently transferred to Kontes homogenization tubes (VWR Scientific Inc, Sugarland, TX) containing 100 to 200 µL of ice-cold lysis buffer (0.5% 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate (CHAPS), 10 mmol/L Tris-HCl [pH 7.5,] 1 mmol/L MgCl2, 1 mmol/L ethyleneglycol-bis-(-aminoethyl ether-N,N,N′,N′-tetraacetic acid (EGTA), 5 mmol/L mercaptoethanol, 0.1 mmol/L [4-(2-aminoethyl)-benzene-sulfonyl fluoride hydrochlorine], 10% glycerol). Homogenization was carried out with disposable pestles (VWR Scientific, Dallas, TX) attached to a cordless drill (Black and Decker, Baltimore, MD) and rotated at 450 rpm. Homogenates were left on ice for 30 minutes, followed by centrifugation of lysates at 14,000 rpm at 4°C for 20 minutes. Supernatants (80–160 µL) were collected in fresh Eppendorf 1.5 micro tubes (United Scientific Products, San Leandro, CA) flash frozen in liquid nitrogen, and stored at -80°C. Before freezing, a 10-µL aliquot was transferred into a separate Eppendorf 1.5 cc micro tube for protein concentration analysis by using the BCA (bicinchoninic acid) protein assay kit (Pierce Chemical Co, Rockford, IL).
Telomeric Repeat Amplification Protocol
To evaluate the approximate levels of telomerase activity in the different dissected compartments, telomeric repeat amplification protocol (TRAP) assays were performed as previously described [30,31], including the use of an internal telomerase assay standard (ITAS) [31] Telomerase can add TTAGGG repeats to a nontelomeric oligonucleotide TS (5′-AATCCGTCGAGCAGAGTT) that serves as the forward primer. These elongation products can then be amplified by polymerase chain reaction (PCR) with the reverse primer CX (5′-[CCCTTA]3 CCCTAA), resulting in a ladder of 6 base pair (bp) addition products.
Tissue extracts were normalized to 6.0 µg total protein per assay. The PCR-based TRAP (Boehringer Mannheim, Indianapolis, IN) assay was carried out in previously prepared HotStart tubes 50 (GIBCO BRL, Gaithersburg, MD) containing 100 ng of CX oligonucleotide beneath a wax barrier and stored at 4°C until needed. Aliquots containing 6.0 µg of total protein were assayed in 50 µL of reaction mixture (20 mmol/L Tris-HCl [pH 8.3], 68 mmol/L KCl, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 0.05% Tween 20, 0.1 µg of TS primer (Midland Certified Reagent Co), 0.5 µmmol/L T4 gene 32 protein, 50 µmol/L of each deoxynucleotide triphosphate, 2 U of Taq DNA polymerase, 4 µCi of [-32P]dCTP [10 µCi/mmol], and 5 x 10-18 g (5 attograms) of ITAS [31] The reaction mixture was incubated at room temperature for 30 minutes to allow telomerase to extend TS primer followed by a 31-cycle PCR amplification (thermocycling block with heated lid, HB-60, MJ Research, Watertown, MA) of the telomeric products. Analysis of 20 µL of the PCR mixtures was performed on 10% nondenaturing acrylamide gels. Gels were fixed in 0.5 mol/L NaCl, 50% ethanol, and 40 mmol/L sodium acetate (pH 4.2) on a rocking table for 30 minutes at room temperature. Excess fixing solution was removed, and gels were exposed at room temperature to phosphor screens overnight and visualized on a PhosphorImager with ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Quantitation of Telomerase Activity
The telomerase assay can be made semiquantitative by the inclusion of 5 x 10-18 g (5 attograms) of an ITAS, a 150-bp fragment containing TS and CX sequences at the ends. That is co-amplified along with the telomerase ladder [30,31]. The levels of telomerase activity were normalized to that of the ITAS. The inclusion of ITAS not only permits relative quantitation between samples, it also aids in the identification of false-negative samples that contain Taq polymerase or other PCR inhibitors. The relative telomerase activity was determined by calculating the ratio of the intensity of the area under peaks representing telomeric repeats (TRAP ladder) to the area under the peak representing amplified ITAS. When the levels of telomerase activity were too high to allow amplification of ITAS, ten-fold dilutions of these samples were assayed for telomerase activity. Triplicate aliquots from selected samples were analyzed, and consistent results were obtained. Pooled ratio results from each sample group were then compared for statistical significance by using nonparametric analyses and rank method (JMP software, Cary, NC). The Wilcoxon/Kruskal Wallis tests combined with the Tukey-Kramer multiple mean comparisons procedure and the ANOVA/t test procedures, were used for all independent groups. All image operations and calculations were performed by using ImageQuant software (Molecular Dynamics) and Microsoft Excel 5.0 (Microsoft Corporation, Redmond, WA).
Preparation and Prehybridization of Tissue Sections for In Situ Hybridization
Formalin-fixed paraffin-embedded (3-µm) tissue sections were placed onto Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). The sections were then deparaffinized, rehydrated in phosphate buffered saline (PBS), and treated with proteinase K (20 µg/mL) in 50 mmol/L Tris-HCl (pH 7.5), 5 mmol/L EDTA, for 20 minutes at room temperature. After rinsing for 5 minutes in PBS, sections were postfixed in 4% paraformaldehyde/PBS, rinsed in water, and acetylated in freshly prepared 0.25% acetic anhydride/0.1 mol/L triethanolamine for 10 minutes. The slides were then dehydrated in gradually increasing concentrations of ethanol before hybridization.
Probe Preparation for In Situ Hybridization
The plasmid pGEM-5Z (Promega Corp, Madison, WI), containing a full-length hTR complementary DNA (560 nucleotides), obtained from the Geron Corp, Menlo Park, CA, was used as a template to generate sense (control) and antisense probe. [35S]UTP-labeled single-stranded RNA probes were synthesized according to manufacturer's (Ambion, Inc, Austin, TX) conditions. Transcripts were alkali hydrolyzed to generate probes, with an average length of 200 nucleotides for efficient hybridization, purified with G-50 column (Boehringer Mannheim Corp, Indianapolis, IN), and precipitated in ethanol. The probes were then resuspended in 30 µL of 100 mmol/L dithiothreitol. The specific activity of the radiolabeled probes, aliquoted and stored at -80°C until use, was approximately 3 to 5 x 106 cpm/µg template DNA.
Hybridization and Washing Procedures
Sections were hybridized overnight at 52°C in 50% deionized formamide, 0.3 mol/L NaCl, 20 mmol/L Tris-HCl (pH 7.5), 5 mmol/L EDTA, 10 mmol/L NaH2PO4 (pH 8.0), 10% dextran sulfate, 1 x Denhardt's, 500 µg/mL total yeast RNA, 10 mmol/L dithiothreitol, and 50,000 cpm/µL 35S-labeled cRNA probe. The slides were then subjected to stringent washing at 50°C in 5 x SSC, 10 mmol/L dithiohreitol for 30 minutes, at 65°C in 50% formamide, 2 x SSC, 10 mmol/L DTT for 30 minutes and washed twice at 37°C in 0.4 mol/L NaCl, 10 mmol/L Tris-HCl (pH 7.5), 5 mmol/L EDTA for 10 minutes before treatment with 20 µg/mL RNase A at 37°C for 30 minutes. Following washes in 2 x SSC and 0.1 x SSC for 1 hour at 37°C, the slides were dehydrated and dipped in Kodak (Eastman Kodak Co., Rochester, NY) NTB-2 nuclear track emulsion and exposed for 3 to 4 weeks in light tight boxes with desiccant at 4°C. The slides were developed in Kodak Dektol developer (3.5 min), washed in water (30 sec), fixed in Kodak fixer (6 min), rinsed in water, counterstained in hematoxylin, dehydrated, coverslips mounted with Paramount (Sigma, St. Louis, MO), and finally observed under light microscope (Olympus America Inc., Lake Success, NY) in both bright and dark fields, followed by photography. In the present study samples were considered hTR positive if uniformly dense silver grains were overlying tumor nuclei similar to those observed in the normal adult testis. Specimens were considered negative if there was complete absence of background hybridization similar to sense control.
Results
Telomerase Activity Is Detected at Highest Levels in Metastases of Melanoma
Sixty-six samples from a total of sixty-three patients with several different types of melanocytic lesions and adjacent skin were examined for telomerase activity (Figure 1 and Table 1). Telomerase activity was detected at much higher levels in subcutaneous metastases of melanoma and metastasized lymph nodes when compared with other melanocytic lesions (Table 1). The mean relative telomerase activity in subcutaneous metastases of melanoma and metastasized lymph nodes was 54.5 and 56.5, respectively, compared with primary melanomas and benign nevi in which relative telomerase activities did not exceed 1.9. This difference between metastatic and nonmetastatic melanomas was statistically highly significant (P value < 0.0001) as determined by the Wilcoxon/Kruskal Wallis test combined with the Tukey-Kramer multiple mean comparison. Although, much lower levels of telomerase activity were detected in primary melanomas (Table 1), even these were significantly elevated when compared with telomerase activity from adjacent control skin samples from the same patient (P values = 0.02, ANOVA/t test). Very low telomerase activity levels were detected in benign melanocytic lesions (e.g., compound and junctional melanocytic nevi), but these were not statistically significant when compared to adjacent control tissues (P values = 0.1809, ANOVA/t test) (Figure 1 and Table 1).
Figure 1.
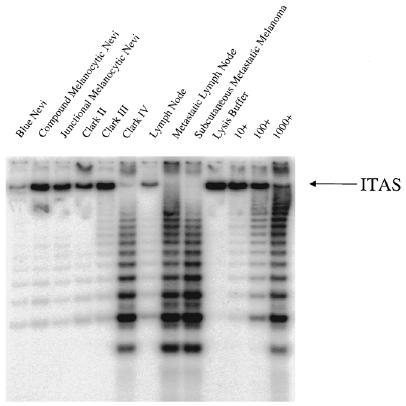
Representative gel of melanocytic specimens for telomerase activity: benign lesions (blue nevi, compound melanocytic nevi, junctional melanocytic nevi); malignant lesions (primary melanoma Clark II, Clark III, and Clark IV); benign lymph node, metastatic lymph node, and a distal subcutaneous metastatic melanoma. Negative control: lysis buffer; positive telomerase controls (a human breast epithelial cell line immortalized with human papilloma virus 16E6/E7): 10 cells, 100 cells, and 1000 telomerase-positive cells. ITAS, a 150-bp fragment containing TS and CX sequences at the ends, co-amplifies with the telomerase ladder. It is included for semiquantitative telomerase activity level analyses and to detect PCR or Taq polymerase inhibitors.
Table 1.
Clinical Stage and Level of Invasion Compared With Telom-erase Activity.
| Number of Specimens | Mean Relative Telomerase Activity | Standard Error | |
| Clinical Stage* | |||
| I–II† | 18 | 1.6 | 0.57 |
| III‡ | 5 | 56.5 | 14.2 |
| IV§ | 8 | 54.5 | 12.3 |
| Benign nevi | |||
| CMN/JMN | 35 | 0.7 | 0.30 |
| Primary melanoma | |||
| Clark I¶ | NA | NA | - |
| Clark II‖ | 1 | 0.02 | - |
| Clark III# | 4 | 1.1 | 1.0 |
| Clark IV** | 13 | 1.9 | 0.7 |
| Metastases of melanoma | |||
| LNM | 5 | 56.5 | 14.2 |
| SCM | 8 | 54.5 | 12.3 |
Staging according to The American Joint Commission on Cancer (AJCC).
Primary melanoma without clinically palpable nodes.
Primary melanoma with clinically palpable nodes.
Patients present with distant metastases.
In situ.
Penetrates papillary dermis.
Fills papillary dermis.
Penetrates reticular dermis.
CMN/JMN, compound melanocytic nevi/junctional melanocytic nevi; NA, not available; LNM, lymph node metastases; SCM, subcutaneous metastases of melanoma.
Telomerase Activity Correlates with the Level of Invasion, Tumor Thickness, and the Mitotic Rate in Malignant Melanoma
Telomerase activity of primary melanomas in cases of clinical stage I and II disease were quantified and compared with the different anatomical levels of invasion (Clark II to IV). The mean relative telomerase activity levels in primary melanoma were as follows: Clark II = 0.02, Clark III = 1.1, and Clark IV = 1.9. Thus, in melanomas that have filled the papillary dermis (Clark III) and penetrated the reticular dermis (Clark IV), telomerase activity levels were higher than in those melanomas with less invasive behavior (Table 1). Telomerase activity was also compared to the Breslow thickness among eighteen primary melanomas (Clark II–IV, clinical stages I and II). Relative mean telomerase activity detected in tumors with thicknesses less than 0.76 mm was 0.2; between 0.76 and 1.69 mm, 2.4; between 1.70 and 3.60 mm, 1.7; and greater than 3.60 mm, 2.5 (Table 2). High mitotic rate lesions had a relative mean telomerase activity of 3.6, whereas low mitotic rate lesions had relative mean telomerase activity of 0.9 (Table 2). These results were statistically significant (P value = 0.03, ANOVA/t test). However, when telomerase levels in radial versus vertical growth patterns were compared, no significant difference is noted (Table 2).
Table 2.
Clinical Features of Primary Melanoma Compared With Telomerase Activity.
| Number of Specimens | Mean Relative Telomerase Activity | Standard Error | |
| Breslow thickness (mm) | |||
| < 0.76 | 6 | 1.2 | 1.00 |
| 0.76–1.69 | 5 | 2.4 | 1.50 |
| 1.70–3.60 | 6 | 1.7 | 0.65 |
| > 3.60 | 1 | 2.5 | - |
| Mitotic rate* | |||
| High | 7 | 3.6 | 0.96 |
| Low | 11 | 0.9 | 0.59 |
| Growth pattern† | |||
| Radial | 2 | 2.0 | 1.75 |
| Vertical | 16 | 1.6 | 0.02 |
| Lymphocyte infiltration‡ | |||
| 0 | 2 | 0.3 | 0.2 |
| 10 | 5 | 2.0 | 1.2 |
| 20 | 2 | 2.7 | 1.3 |
| 50 | 3 | 0.3 | 0.1 |
| 60 | 1 | 4.5 | - |
| 70 | 3 | 2.6 | 2.5 |
| 80 | 1 | 0.03 | - |
High = ≥ 6 mitotic figures per mm2; low = < 6 mitotic figures per mm2.
Radial = horizontal growth (< 1.0 mm); vertical = vertical growth (≥ 1.0 mm).
Expressed in terms of percentage of lymphocytes in the total cell population (100%) constituted by melanocytes and lymphocytes.
Telomerase Activity Compared With the Degree of Lymphocytic Infiltrate
It has been shown previously that leukocytes express low levels of telomerase activity constitutively [32,33] and can increase with antigen stimulation [33]. Furthermore, when the number of leukocytes in skin are increased acutely by inducing dermatitis with cutaneous application of poison ivy antigen, telomerase levels increase compared with normal control skin [29]. This suggests leukocytic infiltration could contribute to the telomerase activity detected in inflammatory conditions of skin, including those associated with malignant melanoma. Lymphocytic infiltrates were carefully quantitated on all samples and then correlated with the level of telomerase activity. No consistent trends were noted (Table 2).
In Situ Telomerase RNA Detection
Because the tissue samples tested for telomerase activity contained varying quantities of nonmelanocytic cells, the possibility exists that their presence could influence the relative amounts of telomerase activity detected (e.g., dilution effect or inhibitory effect). Attempting to use a more sensitive measure of telomerase expression, we undertook an in situ assay for the detection of hTR (Table 3). This had several advantages, including allowing us to use archived formalin fixed paraffin-embedded specimens, and to identify the exact cell type with increased hTR. Previous studies have shown that in situ hTR could easily distinguish cancer cells from normal cells and that the levels of hTR signal correlated well with telomerase activity [20,21]. In situ hTR hybridization evaluation of a Clark's level IV primary melanoma confirmed that the telomerase activity detected in TRAP assays originated from tumor cells because no hTR signals above background levels were observed in lymphocytes or other cell types in the specimens (Figure 2A–I). In areas of tumor cell invasion, large tumor cells with an abundance of cytoplasm were observed to contain hTR-positive nuclei (Figure 2G–H). A pagetoid distribution of large melanoma cells throughout the epidermis (Figure 2A) were clearly hTR positive (Figure 2B–C). Under dark field microscopy, the hTR-positive signals were even more apparent in dermal nodules and intraepidermal collections of malignant cells (Figure 2C, F, and I). All malignant melanomas were positive for hTR, whereas benign lesions (compound/junctional melanocytic nevi, data not shown) were either negative or had near-background levels of hTR in areas where no telomerase activity is typically detected (e.g., dermis or suprabasal layers of epidermis) [29,34].
Table 3.
In Situ Hybridization hTR in Several Melanocytic Conditions.
| Number of Specimens | In Situ hTR* | |
| Benign nevi | ||
| CMN/JMN | 9 | - |
| Primary melanoma | ||
| Clark II† | NA | NA |
| Clark III‡ | 1 | + |
| Clark IV§ | 10 | + |
| Metastases of melanoma | ||
| LNM | 2 | + |
| SCM | 4 | + |
≥ 10 grains over the nucleus = +; < 10 grains over the nucleus = -.
Penetrates papillary dermis.
Fills papillary dermis.
Penetrates reticular dermis.
CMN/JMN, compound melanocytic nevi/junctional melanocytic nevi; NA, not available; LNM, lymph node metastases; SCM, subcutaneous metastases of melanoma.
Figure 2.
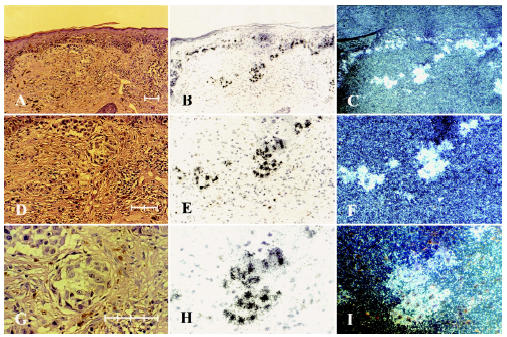
In situ hybridization of primary melanoma Clark IV. A, D and G are stained with hematoxylin and eosin; B, E and H are labeled with radioactive hTR stained with hematoxylin and visualized under bright field microscopy; C, F and I are labeled with radioactive hTR and visualized under dark field microscopy. (A–C are at 100 x final magnification; D – F 200 x final magnification; and G – I 400 x final magnification; Scale bars: A = 80 µm, D = 85 µm, and G = 75 µm).
Discussion
We previously reported telomerase activity is upregulated in skin cancer and that the highest levels were in samples containing basal cell carcinomas [29]. Lower telomerase levels were observed in clinically more malignant tumors such as squamous cell carcinoma and primary cutaneous melanomas [29]. These findings suggested that aggressive behavior did not directly correlate with increased levels of telomerase activity. However, because the number of melanoma samples analyzed in the previous study [29] was small and the lesions were thin, they were thought to be early in their malignant progression. Therefore, the present study was undertaken to evaluate the expression levels of telomerase activity in benign and malignant cutaneous melanocytic lesions with various degrees of invasion. We observed that the highest levels of telomerase activity were detected in metastases of melanoma, whereas the lowest levels of telomerase activity were in benign nevi and low grade melanomas (e.g., Clark II) with little degree of invasion (see Table 1). Regarding Breslow thickness and telomerase activity levels, two groups can be distinguished: those less than 0.76 mm and those greater than 0.76 mm. Melanomas with a Breslow thickness less than 0.76 mm (93% patient survival at eight years) showed telomerase levels 8- to 10-fold lower than those detected in thicker melanomas (< 0.76 mm) known to behave more aggressively (see Table 2). Similarly, telomerase activity levels detected in high mitotic rate lesions were four times higher than those with low mitotic rate lesion (see Table 2). Degree of infiltrating lymphocytes did not correlate with telomerase activity levels, and lymphocytes had very low or failed to express hTR, suggesting that these cells contributed little, if any, to the activity measured (see Table 2). Likewise, no significant difference in telomerase levels was noted between radial and vertical growth (see Table 2). These results suggest that the level of telomerase activity increases with increasing thickness of the lesions, which reflects a greater tumor cell volume and a lesion that has acquired more aggressive properties. Results in this article do not address the mechanism (s) involved in the dramatic upregulation of telomerase activity in metastatic melanoma lesions. Telomerase activity levels in metastatic melanoma are similar to those detected in metastatic lesions of other tumor types [15,29], and possible mechanisms for the increased telomerase activity are transcription upregulation of human telomerase reverse transcriptase—the catalytic subunit of telomerase—(hTERT) or hTERT gene amplification.
Even though hTERT in situ hybridization would be another potential way of detecting the presence of telomerase in cell and tissue samples, alternative splicing variants of hTERT occurs in some telomerase-negative cells, resulting in false-positive signals (data not shown). Thus, to visualize the pattern of telomerase expression among melanomas with different levels of invasion, we used telomerase RNA in situ hybridization techniques. Individual invading melanoma cells were found to be telomerase positive regardless of their location in the skin or lymph node (Table 3). Thus, telomerase RNA may be a useful molecular marker for determining the extent of invasion (e.g., among primary melanomas) and to identify the presence of metastases within lymph nodes and in distant cutaneous tissue. This could potentially improve the accuracy of staging and influence the choice of treatment. However, additional investigations are needed to address the possible correlation of hTR expression level per tumor cell with malignant potential.
Maintaining telomere length stability via expression of telomerase activity in cells may be important in carcinogenesis. Our results suggest that normal nevus cells and melanocytes fail to express telomerase or do so at very low levels. Documenting the correlation of increasing levels of functional telomerase with increasing malignant potential in melanomas strongly suggests that it is not only a feature of malignancy, but quantitation of its activity that may also have clinical prognostic value. Although additional studies must be done to fully characterize the role played by telomerase in melanoma carcinogenesis, it is hoped that the use of new and more efficient telomerase detection assays could potentially assist in the diagnosis of borderline lesions.
Acknowledgements
This work was supported by research grants from the Geron Corporation in Menlo Park, CA (to J.W.S.), the NIH Training Grant T32-GM7062 (to R.D.R., a member of Cell Regulation Graduate Program), and the Italian Ministry of Health (to S.D'A., E.P., T.F., and P.M.L.).
References
- 1.Blackburn EH. Structure and function of telomeres (review) Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW. Mammalian telomere dynamics: Healing, fragmentation shortening and stabilization. Curr Opin Genet Dev. 1994;4:203–211. doi: 10.1016/s0959-437x(05)80046-2. [DOI] [PubMed] [Google Scholar]
- 3.Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201:1496–1499. [PubMed] [Google Scholar]
- 4.Watson JD. Origin of concatemeric T7 DNA. Nature New Biology. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 5.Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 6.Shay JW, Werbin H, Wright WE. Telomere shortening may contribute to aging and cancer: A perspective. Mol Cell Diff. 1994;2:1–18. [Google Scholar]
- 7.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Adams RR, Chang E, Allsopp RC, Yu J, Le S, West MD, Andrews WH, Greider CW, Villeponteau B. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 8.Counter CM, Meyerson M, Eaton EN, Weinberg RA. The catalytic subunit of yeast telomerase. Proc Natl Acad Sci USA. 1997;94:9202–9207. doi: 10.1073/pnas.94.17.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 10.Nakamara TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 11.Allsopp RC, Harley CB. Evidence for a critical telomere length in senescent human fibroblasts. Exp Cell Res. 1995;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- 12.Alberts B, Brays D, Lewis J, et al. In: Molecular Biology of the Cell. 3rd ed. Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD, editors. New York & London: Garland Publishing, Inc; 1994. pp. 1255–1294. [Google Scholar]
- 13.Shay JW, Wright WE. Telomerase activity in human cancer. Curr Opin Oncol. 1996;8:66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Shay JW. Aging and cancer: Are telomeres and telomerase the connection? Mol Med Today. 1995;1:378–384. doi: 10.1016/s1357-4310(95)93872-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 16.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 17.Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995;1:249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 18.Whited JD, Hall RP, Simel DL, Horner RD. Primary care clinicians' performance for detecting actinic keratoses and skin cancer. Arch Intern Med. 1997;157:985–990. [PubMed] [Google Scholar]
- 19.Corona R, Mele A, Amini M, De Rosa G, Coppola G, Piccardi P, Fucci M, Pasquini P, Faraggiana T. Interobserver variability on the histopathologic diagnosis of cutaneous melanoma and other pigmented skin lesions. J Clin Oncol. 1996;14:1218–1223. doi: 10.1200/JCO.1996.14.4.1218. [DOI] [PubMed] [Google Scholar]
- 20.Yashima K, Piatyszek MA, Saboorian HM, Virmani AK, Brown D, Shay JW, Gazdar AF. Expression of telomerase activity and in situ telomerase RNA exression in malignant and non-malignant lymph nodes. J Clin Pathol. 1997;50:110–117. doi: 10.1136/jcp.50.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yashima K, Litzky LA, Kaiser L, Rogers T, Lam S, Wistuba I, Milchgrub S, Srivastava S, Piatyszek MA, Shay JW, et al. Telomerase expression in bronchial epithelium during multistage pathogenesis of lung carcinomas. Cancer Res. 1997;57:2373–2377. [PubMed] [Google Scholar]
- 22.Clark WHJ, Elder DE, Guerry IV D, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 23.Clark WHJ, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–726. [PubMed] [Google Scholar]
- 24.Balch CM, Soong S, Shaw HM, Urist MM, McCarthy WH. In: Cutaneous Melanoma. 2nd ed. Balch CM, Houghton AN, Milton GW, Sober AJ, Soong S, editors. Philadelphia: JB Lippincott; 1992. pp. 165–187. An Analysis of Prognostic Factors in 8500 Patients with Cutaneous Melanoma. [Google Scholar]
- 25.Ketcham AS, Moffat FL, Balch CM. In: Cutaneous Melanoma. 2nd ed. Balch CM, Houghton AN, Milton GW, Sober AJ, Soong S, editors. Philadelphia: JB Lippincott; pp. 213–222. Classification and Staging. [Google Scholar]
- 26.Vollmer RT. Malignant melanoma: A multivariate analysis of prognostic factors. Pathol Annu. 1989;24:383. [PubMed] [Google Scholar]
- 27.Breslow A. Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg. 1975;182:572–575. doi: 10.1097/00000658-197511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172:902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor RS, Ramirez RD, Ogoshi M, Chaffins M, Piatyszek MA, Shay JW. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Invest Dermatol. 1996;106:759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- 30.Piatyszek MA, Kim NW, Weinrich SL, Hiyama K, Hiyama E, Wright WE, Shay JW. Detection of telomerase activity in human cells and tumors by a telomeric repeat amplification protocol (TRAP) Methods Cell Sci. 1995;17:1–15. [Google Scholar]
- 31.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Counter CM, Gupta L, Harley CB, Leber B, Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995;85:2315–2320. [PubMed] [Google Scholar]
- 33.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, Shay JW, Ishioka S, Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 34.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis in human skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci USA. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]


