Abstract
Bcl-2 and Bcl-XL belong to a family of proteins overexpressed in a variety of human cancers which inhibit apoptosis in response to a number of stimuli including chemotherapeutic agents and ionizing radiation. To better understand the role of these polypeptides in modulating the response of cancer cells to ionizing radiation we used cell lines that were engineered to overexpress the two polypeptides. Although Bcl-2 and Bcl-XL overexpression resulted in inhibition of radiation-induced apoptosis, it did not result in enhanced clonogenic survival. Consistent with this was the observation that Bcl-2 and Bcl-XL protected cells from DNA fragmentation, loss of mitochondrial membrane potential, and caspase activation for up to 72 hours after irradiation. Beyond 72 hours, there was a rapid loss in the ability of Bcl-2 and Bcl-XL to inhibit these markers of apoptosis. When Bcl-XL was analyzed at 72 hours after irradiation and beyond, a rapid accumulation of a 16-kDa form of Bcl-XL was observed. To test the hypothesis that cleavage of the 29-kDa form of Bcl-XL by caspases to a 16-kDa polypeptide results in its inability to inhibit apoptosis beyond 72 hours, we constructed a cell line that overexpressed a caspase-resistant form of Bcl-XL Bcl-XLΔloop. Cells overexpressing Bcl-XL-Δloop were resistant to apoptosis beyond 72 hours after irradiation and did not contain the 16-kDa form at these time points. In addition, Bcl-XL-Δloop overexpression resulted in enhanced clonogenic survival compared with control or Bcl-XL overexpressing cells. These results provide a molecular basis for the observation that expression of Bcl-2 or Bcl-XL is not a prognostic marker of tumor response to cancer therapy.
Keywords: apoptosis, ionizing radiation, Bcl-X, caspase, survival
Introduction
Eukaryotic cells respond to ionizing radiation by undergoing cell cycle arrest to allow for DNA repair. In the event of irreparable damage, irradiated cells can undergo programmed cell death or apoptosis. Our understanding of the molecular events that lead to activation of the apoptotic pathway in response to ionizing radiation is limited, although numerous studies have demonstrated a requirement for p53 for efficient apoptosis [1,2] and the activation of zymogen caspases as an essential step in radiation-induced apoptosis [3]. In addition, consistent with their function in regulating apoptosis in response to various insults, overexpression of Bcl-2 [4] and Bcl-XL [5] results in inhibition of radiation-induced apoptosis. Two primary activities have been assigned to Bcl-2 and Bcl-XL in their function as inhibitors of apoptosis. First, Bcl-2 and Bcl-XL have been shown to be able to inhibit the activation of zymogen caspases [6,7], and second, they are able to protect the loss of mitochondrial membrane potential [8] in response to various apoptotic stimuli. Although apoptosis has been identified as an important determinant of tumor growth as well as of response to cancer therapy [9], the role of Bcl-2 and Bcl-XL expression levels as predictive markers of clinical responsiveness is unclear. Reports that Bcl-2 expression is indicative of a poor prognosis have been contradicted by those that suggest an improved prognosis by the presence of Bcl-2 overexpression [10–12]. Additionally, in-vitro studies on the role of Bcl-2 in regulating sensitivity of cell lines to apoptosis induced by chemotherapeutic agents as well as ionizing radiation confirm the ability of Bcl-2 to inhibit apoptosis but fail to demonstrate enhanced long-term survival of such cell lines [13–15].
To better understand the role of Bcl-2 and Bcl-XL in cancer therapy, we used lymphoid as well as breast carcinoma cells that were engineered to overexpress these two proteins. Overexpression of Bcl-2 and Bcl-XL resulted in inhibition of apoptosis induced by irradiation, but failed to promote clonogenic survival. These results were consistent with the observation that Bcl-2 and Bcl-XL only inhibited apoptosis for up to 72 hours after irradiation, after which there was a time-dependent loss in protection from apoptosis. Coincident with the inability of Bcl-XL to protect cells from apoptosis, there was an appearance of active caspase 3 and cleavage of Bcl-XL to a 16-kDa form. Bcl-XL-Δloop (a caspase-resistant form of Bcl-XL), on the other hand, protected cells from apoptosis beyond 72 hours and was also able to enhance clonogenic survival. These studies provide a molecular explanation for the often made observation that Bcl-2 and Bcl-XL only delay the onset of apoptosis.
Materials and Methods
Cell Lines and Culture Conditions
The MCF7 and Jurkat stable transfected cell lines (Bcl2 and Bcl-XL) used in this study have been described previously [16]. The cells were maintained in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 1% L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37° in an atmosphere of 5% carbon dioxide. The transfected Jurkat cell lines (Bcl2 and Bcl-XL) were grown in the presence of 0.4 mg/mL Hygromycin (Boehringer Mannheim, Indianapolis, IN). Wild-type Jurkat cells were treated with 25 µmol/L ZVAD-fmk (Enzyme System Products, Livermore, CA) 24 hours before irradiation and again after it.
Irradiations
Freshly seeded Jurkat cells were irradiated at room temperature with a 60Co source at 1 to 2 Gy/minutes. Dositmetry was carried out with an ionization chamber connected to an electrometer system that was directly traceable to a NIST standard. After irradiation, the cells were incubated for the indicated time.
Cell Survival Assay
Cell survival was assessed with a standard clonogenic assay. The survival curves were fitted by using a linear-quadratic equation. The surviving fraction was calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose after radiation exposure.
Trypan Blue Assay
Acute viability was assessed by using trypan blue solution (0.4%) (Sigma, St. Louis, MO) diluted in phosphate-buffered solution (PBS) after irradiation (10 Gy) at the indicated timepoints. A minimum of 300 cells were counted for each sample, and the results were based on 4 independent experiments.
Morphological Analysis
Apoptotic morphology was assessed by using a DNA 6staining dye. Jurkat cells (1–2 x 106) were stained with propidium iodide (Sigma, St. Louis, MO). After irradiation both treated and control cells were incubated for either 72 hours or 168 hours before staining. Cells were rinsed 2 times with PBS, fixed in 4% paraformaldehyde at room temperature for 30 minutes, rinsed with PBS, and stained at room temperature for 30 minutes in a 50 µg/mL solution of propidium iodide in PBS. After staining, the cells were rinsed 3 times with PBS, resuspended in a small volume of PBS, 15 µL aliquots were transferred to glass slides, and cells were mounted by using Vectashield mounting medium (Vector Laboratories, Buringame, CA). Slides were examined immediately after staining with a Leitz Laborlux S microscope. Apoptotic cells were quantitated based on nuclear morphology by using fluorescence microscopy, and the percentage of apoptotic cells was calculated. A minimum of 200 cells was counted for each sample, and each experiment was done at least in duplicate.
Flow Cytometry
Jurkat cells were irradiated with 10 Gy of gamma radiation. At the indicated time points, 2 x 106 cells were harvested, washed with PBS, fixed by dropwise addition of ice cold 70% ethanol, and stored at 4 degrees until the day of analysis. The cells were pelleted, washed with PBS, and stained with propidium iodide (final concentration 20 ug/ml PI and 40 ug/ml Ribonuclease A in PBS). The samples were analyzed on a Coulter XL flow cytometer (Coulter Electronics, Hialeah, FL). Trout erythrocyte nuclei were used as an internal standard (Biosure).
Mitochondrial Membrane Potential Assay
Jurkat cells 5 x 106 were either left untreated or treated with 10 Gy of gamma radiation each day for the indicated time points starting with the longest incubation (168 hours). On the day of analysis, 2 x 106 cells for each sample were removed, pelleted, and resuspended in 1 to 2 mL of PBS buffer containing 5 µg/mL rhodamine 123 (Molecular Probes, Eugene, OR). Cells were incubated for 30 min at 37°, washed with PBS, and resuspended in 0.5 mL of PBS buffer containing 2 µg/mL propidium iodide. The samples were analyzed immediately on a Coulter XL flow cytometer.
Bcl-XL Immunoblotting
In each sample, 15 x 106 cells were either left untreated or treated with 10 Gy of gamma radiation. At the indicated timepoints (72, 144, and 192 hours) cells were pelleted, washed with PBS, and lysed with NP-40 lysis buffer containing a protease inhibitor mixture Boehringer Mannheim, Indianapolis, IN. The lysates were assayed for protein concentrations the Bio-Rad Dc protein assay kit (Bio-Rad Laboratories, Hercules, CA). After quantitation, an equal volume of Laemmli buffer was added to each sample. The extracts were then boiled for 5 minutes and 100 µg of protein was loaded and resolved on a 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The resolved samples were transferred onto a nitrocellulose membrane (Gelman Sciences, Ann Arbor, MI) for Western blot analysis. The presence of Bcl-XL cleaved and uncleaved products was detected by using a rabbit polyclonal antibody (1:500 dilution) against Bcl-X (Bcl-XS/L (L-19), Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and a horseradish peroxidase (HRP)-conjugated secondary antibody. The blots were developed by using a CL-HRP substrate system (Pierce, Rockford, IL).
Results
Bcl-2 and Bcl-XL Inhibit Radiation-Induced Apoptosis but Fail to Promote Long-Term Survival
To understand the contribution of apoptosis in radiation-induced cell death we first investigated the impact of Bcl-2 and Bcl-XL expression on clonogenic survival of Jurkat cells after ionizing radiation. In contrast to our initial prediction, expression of Bcl-2 or Bcl-XL did not significantly alter the clonogenic survival of Jurkat cells (Figure 1A). Similarly, treatment of Jurkat cells with the caspase inhibitor ZVAD-fmk also had no significant impact on clonogenic survival (Figure 1A) compared with untreated Jurkat cells. To examine if this was a cell line-specific phenomenon, we studied clonogenic survival of MCF-7 cells in the presence or absence of Bcl-XL expression. As shown in Figure 1B, Bcl-XL expression did not protect MCF-7 cells from radiation-induced clonogenic death. To test if the failure to enhance clonogenic survival under these conditions was due to failure of Bcl-2, Bcl-XL, and ZVAD-fmk to inhibit radiation-induced cell death, we performed a short-term viability experiment. Control Jurkat cells, Bcl-2 or Bcl-XL expressing Jurkat cells, and ZVAD-fmk-treated Jurkat cells were irradiated, after which cell viability was measured by trypan blue exclusion. Expression of Bcl-2 or Bcl-XL, as well as treatment with the caspase inhibitor ZVAD-fmk, resulted in significant protection from radiation-induced cell death in a dose-dependent manner, compared with untreated cells (Figure 1C).
Figure 1.
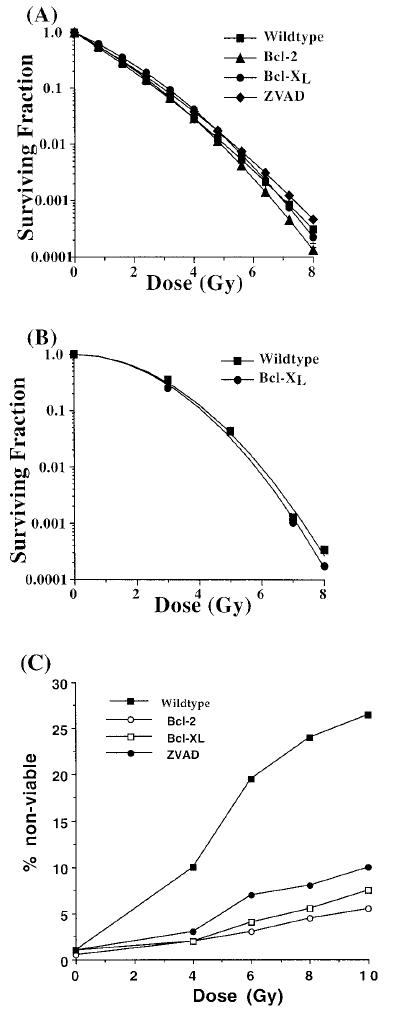
Overexpression of Bcl-2 and Bcl-XL and ZVAD-fmk treatment promotes cell viability 48 hours after irradiation but fails to enhance clonogenic survival. A Bcl-XL (circles) or Bcl-2 (triangles) overexpressing Jurkat cells, as well as ZVAD-fmk-treated control (diamonds) and untreated control (squares) Jurkat cells were treated with various doses of ionizing radiation. The fraction of cells surviving the treatment was determined by plating a fixed number of cells onto dishes to allow for colony formation. The data presented are derived from a single experiment. Similar results were obtained from at least 3 independent experiments. B Wild-type MCF-7 (squares) cells or Bcl-LX overexpressing MCF-7 cells (circles) were irradiated with various doses of ionizing radiation, and the surviving fraction of cells was determined by plating a fixed number of cells onto dishes to allow for colony formation. C Wild-type Jurkat cells (closed squares), as well as Bcl-2 (open circles) or Bcl-XL (open squares) overexpressing and ZVAD-fmk-treated Jurkat cells (closed circles) were treated with various doses of ionizing radiation, and cell viability was determined by trypan blue exclusion 48 hours after irradiation.
We hypothesized that the discrepancy in these two results was due to the clonogenic survival assay requirement of a 10 to 14 day period, in which a surviving cell has to form a colony. However, in the viability assay, survival is determined immediately by using trypan blue exclusion. To further examine if Bcl-XL and Bcl-2 are able to inhibit apoptosis in the short term but not in the long term, cells were irradiated with 10 Gy, and the fraction of cells appearing morphologically apoptotic (as visualized by the presence of condensed chromatin and pyknotic nuclei) after 72 hours or 168 hours was counted. As shown in Figure 2A, 22% of Jurkat cells appeared morphologically apoptotic 72 hours after irradiation, whereas Jurkat/Bcl-2 and Jurkat/Bcl-XL cells appeared 4% and 3% apoptotic, respectively. ZVAD-fmk treated cells were 10% apoptotic 72 hours after irradiation. When the same cultures were analyzed for morphological apoptosis 168 hours after irradiation (Figure 2B), Jurkat cells appeared 45% apoptotic, whereas Bcl-2 and Bcl-XL overexpressing cells appeared 25% apoptotic (Figure 2B) and ZVAD-fmk treated cells were 30% apoptotic. The ability of Bcl-2 and Bcl-XL to inhibit apoptosis by greater than 5-fold at the 72-hour timepoint and by less than 2-fold at the 168-hour timepoint is consistent with our hypothesis that Bcl-2 and Bcl-XL inhibit apoptosis in the short term but not in the long term.
Figure 2.
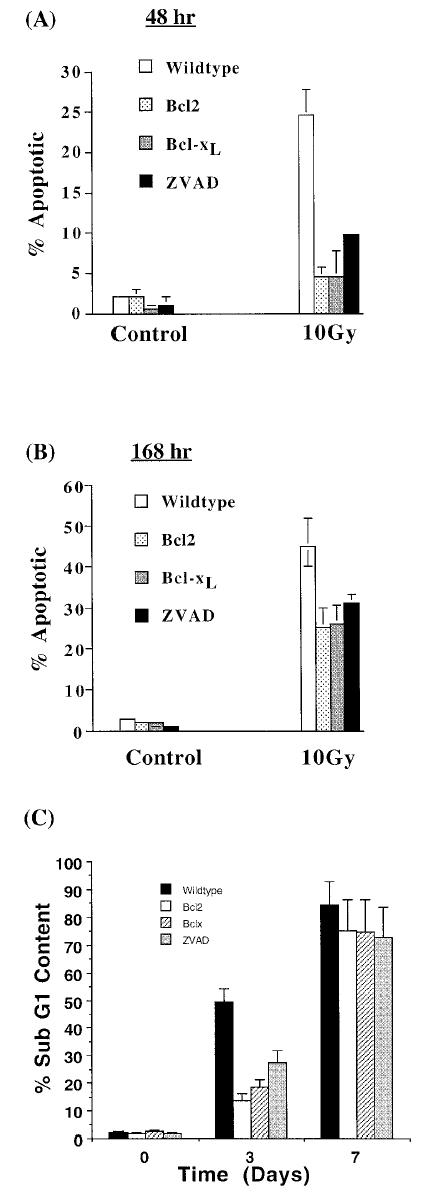
Overexpression of Bcl-2 and Bcl-XL and ZVAD-fmk treatment inhibits apoptosis at 48 hours but not at 168 hours after irradiation. Bcl-2 or Bcl-XL overexpressing, ZVAD-fmk-treated, or untreated, wild-type Jurkat cells received 10 Gy of ionizing radiation, and the appearance of apoptotic morphology was examined after 48 hours (A) and after 168 hours (B). These data were averaged from 3 independent experiments with the bars representing standard error. In parallel experiments, the presence of apoptotic cells was identified by using propidium iodide staining of ethanol-fixed cells, followed by flow analysis. The appearance of cells having a less than G1 DNA content due to DNA fragmentation was averaged from 3 experiments (C).
Bcl-2 and Bcl-XL Delay but Do Not Prevent DNA Fragmentation, Loss of Mitochondrial Membrane Potential, and Caspase-3 Activation
The results just discussed were substantiated when hypodiploidy was used as a measure of apoptosis (Figure 2C). Bcl-2 and Bcl-XL overexpression as well as ZVAD-fmk treatment resulted in inhibition of DNA fragmentation (as determined by the presence of cells having a less than G1 content of DNA), 3 but not 7 days after irradiation. Bcl-XL and Bcl-2 mediate their antiapoptotic effect by 2 mechanisms. First, Bcl-XL protects the loss of mitochondrial membrane potential, and second, Bcl-XL inhibits activation of caspases. To test if the loss of Bcl-XL's ability to inhibit radiation-induced apoptosis at day 7 was consistent with the loss of these functions, we performed the following experiments. Using rhodamine 123 fluorescence as a marker for mitochondrial membrane potential and propidium iodide staining as marker for cell membrane integrity (and therefore cell viability), we demonstrated that 45% of Jurkat cells lost their mitochondrial membrane potential (as evidenced by a decrease in Rh123 fluorescence) and were nonviable (as evidenced by increased propidium iodide staining) 72 hours after irradiation (Figure 3A and B). Bcl-XL overexpression resulted in only 17% of Jurkat/Bcl-XL cells losing their mitochondrial membrane potential at day 3 (Figure 3A and B). Similarly, Bcl-2 expression also inhibited radiation-induced loss of mitochondrial membrane otential and apoptosis 3 days after irradiation. The ability of Bcl-2 and Bcl-XL to protect mitochondrial dysfunction was lost beyond day 6. ZVAD-fmk treatment also resulted in inhibition of loss of mitochondrial membrane potential, although to a much lesser extent. Interestingly, Bcl-2 inhibited apoptosis as late as day 5, at which point Bcl-XL and ZVAD-fmk had lost their inhibitory capacity (Figure 3B).
Figure 3.
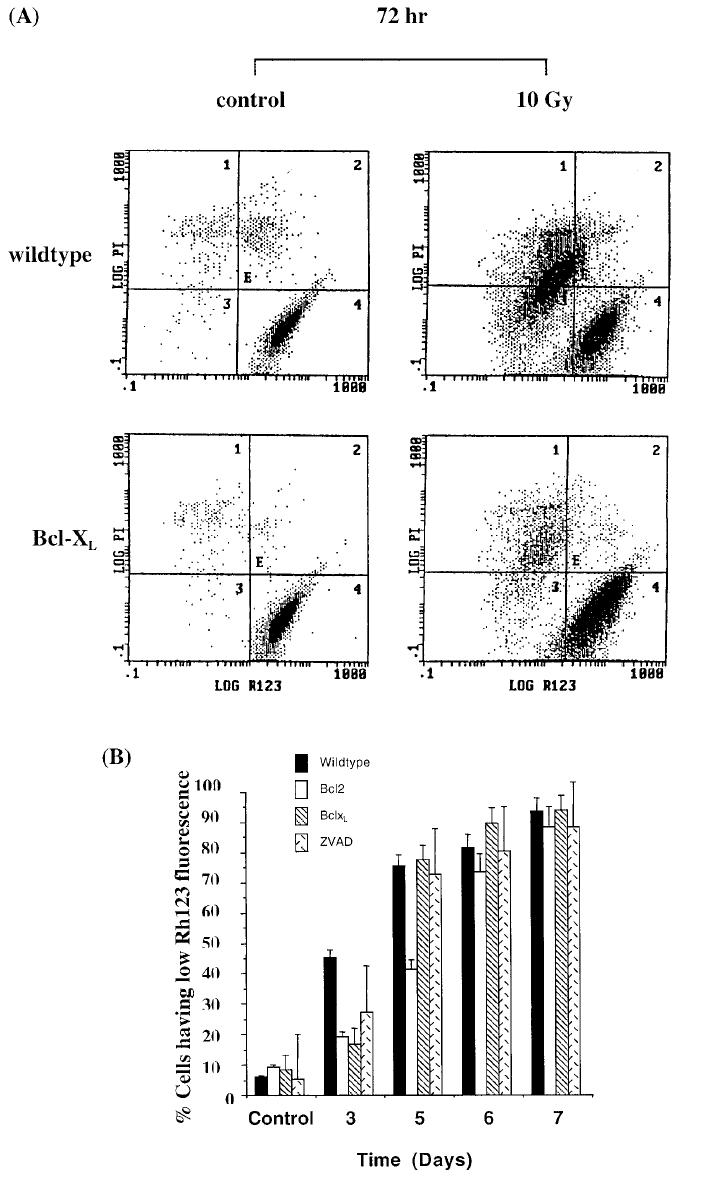
Bcl-2 and Bcl-XL expression protects loss of mitochondrial membrane potential at 3 but not at 6 days after ionizing radiation. Bcl-XL overexpressing or wild-type Jurkat cells were irradiated with 10 Gy of ionizing radiation, after which the cells were stained with Rh123 and propidium iodide and analyzed by flow. A. A decrease in Rh123 fluorescence (leftward shift on the X-axis) is indicative of loss of mitochondrial membrane potential, whereas an increase in propidium iodide staining (upward shift on the Y-axis) is indicative of loss of cell membrane integrity and hence viability. B. Data from 3 independent experiments were averaged (± standard error). Wild-type Jurkat cells are shown by solid bars, Bcl-2 overexpressing cells with open bars, Bcl-XL expressing cells with cross bars, and ZVAD-treated wild-type cells with double bars.
Recent reports have suggested that cleavage of Bcl-2, as well as Bcl-XL, by caspases can result in loss of function [17–19]. To examine if loss of Bcl-XL function at the later timepoints in response to ionizing radiation correlated with its cleavage, we performed Western blot analysis of Jurkat/Bcl-XL cells. Untreated cells or irradiated cells were harvested at the indicated timepoints and analyzed. As shown in Figure 4A, 72 hours after irradiation an additional Bcl-XL immunoreactive band was detected at 16 kDa. This form of Bcl-XL was undetectable in untreated cells Figure 4A, as well as 24 hours and 48 hours after irradiation (data not shown). The 16-kDa band accumulated in a time-dependent manner. Interestingly, detection of the 16-kDa form of Bcl-XL coincided with the appearance of active caspase 3 (Figure 4B). Caspase 3 is a zymogen that is cleaved to a 2-chain polypeptide during apoptosis, resulting in its functional activation. The cleaved form of caspase 3 was undetectable in nonirradiated cells (Figure 4B) or at 24 and 48 hours after irradiation of Jurkat/Bcl-XL cells (data not shown). Cleaved, active caspase was readily detectable 72 hours after irradiation and accumulated at 144 and 192 hours. The appearance of the 16-kDa form of Bcl-XL mirrored the activation of caspase 3, suggesting that activation of caspase 3 resulted in cleavage of Bcl-XL to a 16-kDa protein. Taken together, these results indicate that expression of Bcl-XL results in inhibition of radiation-induced apoptosis in the short term (up to 72 hours), but subsequent cleavage and inactivation of Bcl-XL results in loss of protection from apoptosis.
Figure 4.
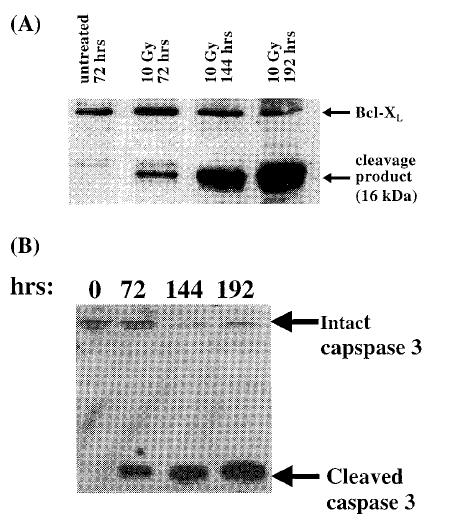
Cleavage of Bcl-XL and caspase 3 activation is initiated 72 hours after irradiation. Bcl-XL overexpressing Jurkat cells were irradiated with 10 Gy of ionizing radiation, and cell extracts were prepared at various times and analyzed by Western blot for the presence of Bcl-XL cleavage (A) and caspase 3 activation (B).
Role of Caspases in the Cleavage and Inactivation of Bcl-XL
To directly test the role of caspases in the inactivation of Bcl-XL, we used a mutant of Bcl-XL that lacks the caspase recognition sequence (Bcl-XL-Δloop) [20]. Bcl-XL-Δloop cannot be cleaved but still retains antiapoptotic function. Jurkat cells were stably transfected with the expression vector for Bcl-XL-Δloop to derive the Jurkat/Bcl-XL-Δloop cell line. When cell extracts from irradiated Jurkat/Bcl-XL and Jurkat/Bcl-XL-Δloop cells were analyzed by Western blot, Bcl-XL was detected as a 29-kDa polypeptide at the 24-and 48-hour timepoints (data not shown). As before, Bcl-XL was also detected as a 16-kDa polypeptide 72 and 144 hours after irradiation (but not in the absence of irradiation), whereas Bcl-XL-Δloop was not cleaved to the 16-kDa L species at these times after irradiation (Figure 5A). Next, we examined the ability of Bcl-XL-Δloop to protect cells from cell death at different times after irradiation compared to wild-type Bcl-XL. As shown in Figure 5B, Bcl-XL-loop, unlike wild type Bcl-XL, was able to protect cells from radiation-induced cell death at early (24 and 48 hours) as well as late timepoints (beyond 72 hours). The ability of Bcl-XL-Δloop to protect cells from radiation-induced cell death also resulted in enhanced clonogenic survival of cells expressing the caspase-resistant mutant of Bcl-XL, compared with cells expressing the wild-type molecule or cells that were transfected with the vector only (Figure 6).
Figure 5.
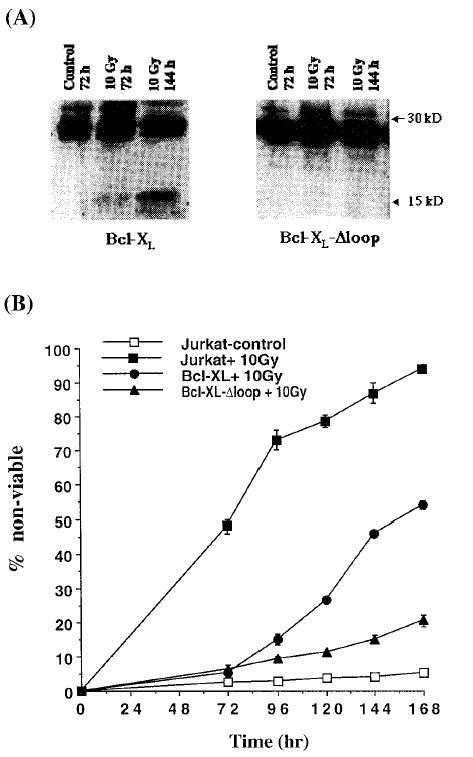
Bcl-XL-Δloop but not Bcl-XL is resistant to cleavage by caspases and is able to inhibit apoptosis in the short- and long-term assays. Jurkat cells overexpressing Bcl-XL or Bcl-XL-Δloop were irradiated with 10 Gy of ionizing radiation, and at various times cells were collected for the preparation of cell extracts for Western blot analysis with a Bcl-XL-specific antibody (A). At these times cells were also collected for determination of cell viability with trypan blue exclusion (B). Control cultures that were mock irradiated were also analyzed in parallel. Data presented in (B) are an average of 4 experiments, with bars representing standard error.
Figure 6.
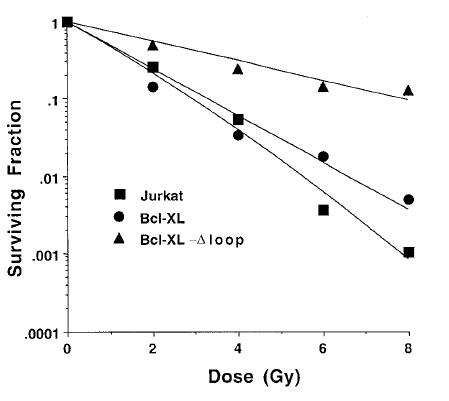
Bcl-XL-Δloop-expressing Jurkat cells have an improved clonogenic survival in response to ionizing radiation, compared with Jurkat cells or Jurkat/Bcl-XL cells. Jurkat cells (squares), Jurkat/Bcl-XL cells (circles), or Jurkat/Bcl-XL-Δloop cells (triangles) were irradiated with various doses of ionizing radiation and immediately plated onto dishes for the determination of the surviving fraction. Bcl-XL-Δloop-overexpressing cells were much more resistant to ionizing radiation and resulted in a much larger fraction of surviving cells at all doses of radiation. Similar results were obtained from 3 independent experiments, and the data presented are from a representative experiment.
Discussion
Our studies on understanding the role Bcl-2 and Bcl-XL play in determining the radiosensitivity of tumor cells led to the observation that expression of these antiapoptotic proteins results in inhibition of apoptosis when assays that measure apoptosis within 72 hours after irradiation are used. In contrast, when apoptosis was measured 96 hours or more after irradiation or with either a clonogenic assay, a morphological assay, or a cell viability assay, the ability of Bcl-2 and Bcl-XL to inhibit radiation-induced apoptosis was greatly diminished. Inability of these proteins to block apoptosis in the long term has been observed in response to many stimuli. Bissonnette et al. [21] observed that in Chinese hamster ovary cells undergoing c-myc-induced apoptosis, Bcl-2 expression resulted in a temporary delay but did not inhibit apoptosis. Yin and Schimke [14] also observed that Bcl-2 expression inhibited apoptosis in response to colecimid, aphidicolin, and trimetrexate treatment when short-term assays of apoptosis were used, such as determination of nuclear morphology after propidium iodide staining and vital dye exclusion. In contrast, when a clonogenic assay was used to measure cell viability, Bcl-2 expression did not appear to protect cells from these insults. Kyprianou et al. [13] also observed that Bcl-2 expression rendered prostate cancer cells resistant to radiation-induced apoptosis but failed to enhance clonogenic survival. A number of possible explanations for the discrepancy in the role of Bcl-2 in inhibiting apoptosis by clonogenic assays compared with short-term assays have been put forward. Yin and Schimke [14], as well as Kyprianu et al. [13], suggested that this inconsistency could be due to the fact that Bcl-2 may not inhibit apoptosis but simply delay it. Milner et al. [22] concluded that Bcl-2 expression results in inhibition of apoptosis in irradiated B-lymphoma-derived cells when short-term assays but not long-term assays are used, because Bcl-2 can also promote apoptosis through its ability promote growth arrest. Lock and Stribinskiene [15] proposed that the ability of Bcl-2 to prevent apoptosis may not translate into increased survival in response to etoposide treatment because Hela cells respond to etoposide either by apoptosis or by mitotic catastrophe. Cells that are protected from apoptosis by Bcl-2 expression fail to survive in the long term owing to mitotic death.
To understand the molecular basis of the failure of Bcl-2 and Bcl-XL to protect cells from undergoing apoptosis at later timepoints (beyond 96 hours), we examined the key activities of these proteins over time. Two primary activities have been ascribed to Bcl-2 and its homologues, such as Bcl-XL. First, in an Apaf-1-dependent manner, Bcl-2-like proteins can inhibit activation of caspases [23]. Second, the ability of Bcl-2-like proteins to inhibit loss of mitochondrial membrane potential is also key to their function [23]. Our results indicate that despite the presence of Bcl-XL, irradiation of Jurkat cells resulted in activation of caspase 3 at 72 hours. In addition, despite the presence of Bcl-2 and Bcl-XL, irradiation of Jurkat cells resulted in the loss of mitochondrial membrane potential, although in a much more delayed time course compared with control cells. Inability of Bcl-2 or Bcl-XL to protect cells from apoptosis in the long term is consistent with the inability of these proteins to completely inhibit caspase activation or to protect the loss of mitochondrial membrane potential in the long term. This, therefore, suggested to us that Bcl-2 and Bcl-XL must be nonfunctional at these timepoints. Based on previous observations [17–19] that both these proteins can be cleaved and inactivated during apoptosis, we investigated whether during radiation-induced apoptosis, cleavage of Bcl-XL was occurring. Indeed, approximately 72 hours after irradiation, a 16-kDa form of Bcl-XL was detected in cell extracts, and this continued to accumulate with time. Analysis of Bcl-2 also revealed the appearance of an approximately 22-kDa polypeptide at 96 hours after irradiation (data not shown). Appearance of the cleaved form of Bcl-XL and Bcl-2 coincided with their inability to protect cells from radiation-induced apoptosis. In addition, cleavage of Bcl-XL was coincident with the presence of active caspase 3 at 72 hours. This is consistent with published results that the 16-kDa form of Bcl-XL appears upon cleavage of Bcl-XL at an aspartic acid residue at position 61 [17]. The report also demonstrated that the 16-kDa form of the protein, which lacks the BH4 domain, has proapoptotic activity rather than antiapoptotic activity, similar to Bcl-XS. We believe that in our system, in response to the appearance and accumulation of the cleaved protein, there was a corresponding increase in the rate of apoptosis.
Recent reports that the cleavage of Bcl-2 by caspases also results in a polypeptide with proapoptotic activity [18] further validates our hypothesis that failure to protect cells from radiation-induced apoptosis in the long term is due to cleavage of antiapoptotic proteins such as Bcl-2 and Bcl-XL. To test the role of caspases in inactivating Bcl-XL, we irradiated Bcl-XL expressing and nonexpressing Jurkat cells and monitored cell viability over time in the presence or absence of caspase inhibitors. Although the expression of Bcl-XL protected Jurkat cells from cell death over the first 72 hours, we consistently observed an acceleration in the rate of apoptosis beyond this time, which we propose is due to inactivation of Bcl-XL by caspases. This acceleration in the rate of apoptosis was greatly inhibited in the presence of caspase inhibitors (ZVAD-fmk, data not shown), suggesting that inhibition of caspases prevented the cleavage of Bcl-XL, which therefore retained its antiapoptotic function. To directly test the role of caspases in the cleavage and inactivation of Bcl-XL, a deletion mutant that lacks a caspase recognition sequence and hence a noncleavable form of Bcl-XL (Bcl-XL-Δloop) was used [20]. Expression of Bcl-XL-Δloop resulted in protection of cells from apoptosis in the short term as well as long term and also enhanced clonogenic survival. These studies provide a definitive explanation for a number of previous observations that indicated that the antiapoptotic proteins Bcl-2 and Bcl-XL simply delay the induction of apoptosis.
We believe our results also provide a molecular explanation for numerous studies that failed to demonstrate a correlation between expression of antiapoptic proteins such as Bcl-2 in tumors and a poor clinical prognosis. In some reports, expression of Bcl-2 was associated with improved prognosis [10–12]. Based on previous reports that the caspase cleaved form of Bcl-2 has proapoptotic activity and based on results presented here, it is not difficult to imagine how a Bcl-2 overexpressing tumor would respond better to therapy. Further studies to test the impact of Bcl-2, Bcl-XL, and Bcl-XL-Δloop expression on tumor response during radiation treatment as well as during chemotherapy are in progress and will enable the evaluation of the role of Bcl-XL cleavage in vivo.
Acknowledgements
We thank Amy Pace for the preparation of the figures, Mary Davis for her support throughout these studies. This work was supported by NIH grants CA78041 (A.R.) and CA56663 (J.M.) J.F. is supported by a Giffum Upjohn Fellowship and training grant GM07767.
References
- 1.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;62:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 2.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper MI, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 3.Datta R, Kojima H, Banach D, Bump NJ, Talanian RV, Alnemri ES, Weichselbaum RR, Wong WW, Kufe DW. Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis. J Biol Chem. 1997;272:1965–1969. doi: 10.1074/jbc.272.3.1965. [DOI] [PubMed] [Google Scholar]
- 4.Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 5.Datta R, Manome Y, Taneja N, Boise LH, Weichselbaum R, Thompson CB, Slapak CA, Kufe D. Overexpression of Bcl-XL by cytotoxic drug exposure confers resistance to ionizing radiation-induced internucleosomal DNA fragmentation. Cell Growth Differ. 1995;6:363–370. [PubMed] [Google Scholar]
- 6.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamen R, Weichselbaum R, Kufe D. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan G, O'Rourke K, Dixit VM. Caspase 9, Bcl-XL and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 8.Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson CB. Apoptosis in pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 10.Pezzella F, Jones M, Ralfkiaer E, Ersboll J, Gatter KC, Mason DY. Evaluation of bcl-2 protein expression and 14;18 translocation as prognostic markers in follicular lymphoma. Br J Cancer. 1993;65:87–89. doi: 10.1038/bjc.1992.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piris MA, Pezzella F, Martinez-Montero JC, Orradre JL, Villuendas R, Sanchez-Beato M, Cuena R, Cruz MA, Martinez B, et al. p53 and bcl-2 expression in high-grade B-cell lymphomas: correlation with survival time. Br J Cancer. 1994;69:337–341. doi: 10.1038/bjc.1994.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjalma W, Weyler J, Goovaerts G, De Pooter C, Van Marck E, van Dam P. Prognostic value of bcl-2 expression in patients with operable carcinoma of the uterine cervix. J Clin Pathol. 1997;50:33–36. doi: 10.1136/jcp.50.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyprianou N, King ED, Bradbury D, Rhee JG. bcl-2 over-expression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer. Int J Cancer. 1997;70:341–348. doi: 10.1002/(sici)1097-0215(19970127)70:3<341::aid-ijc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Yin DX, Schimke RT. BCL-2 expression delays drug-induced apoptosis but does not increase clonogenic survival after drug treatment in HeLa cells. Cancer Res Nov. 1995;55:4922–4928. [PubMed] [Google Scholar]
- 15.Lock RB, Stribinskiene L. Dual modes of death induced by etoposide in human epithelial tumor cells allow Bcl-2 to inhibit apoptosis without affecting clonogenic survival. Cancer Res. 1996;56:4006–4012. [PubMed] [Google Scholar]
- 16.Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 17.Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci USA. 1997;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;1997(278):1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 19.Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C, Michel MR. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: Evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998;17:1268–1278. doi: 10.1093/emboj/17.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson CB. Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissonnette RP, Echeverri F, Mahboubi A, Green DR. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;59:552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- 22.Milner AE, Grand RJ, Vaughan AT, Armitage RJ, Gregory CD. Differential effects of BCL-2 on survival and proliferation of human B- lymphoma cells following gamma-irradiation. Oncogene. 1997;15:1815–1822. doi: 10.1038/sj.onc.1201355. [DOI] [PubMed] [Google Scholar]
- 23.Adams JM, Cory S. The Bcl-2 protein family: Arbiters of cell survival. Science. 1998;28:11322–11326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]


