Abstract
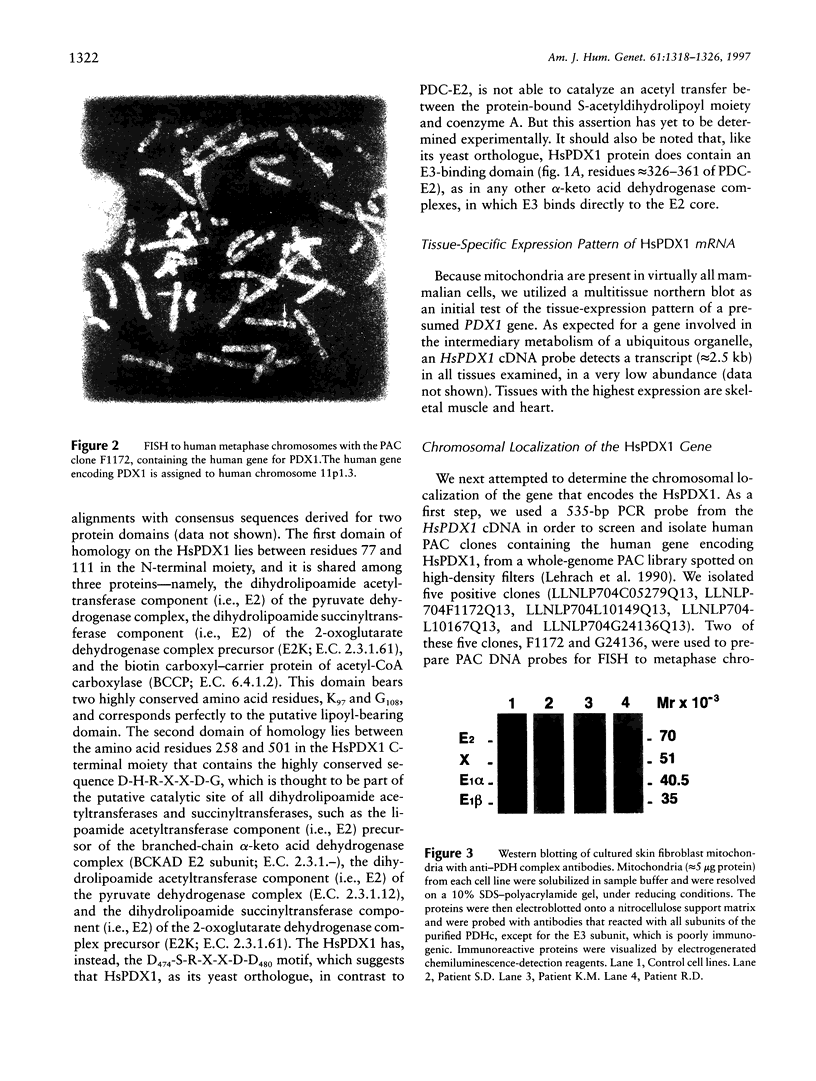
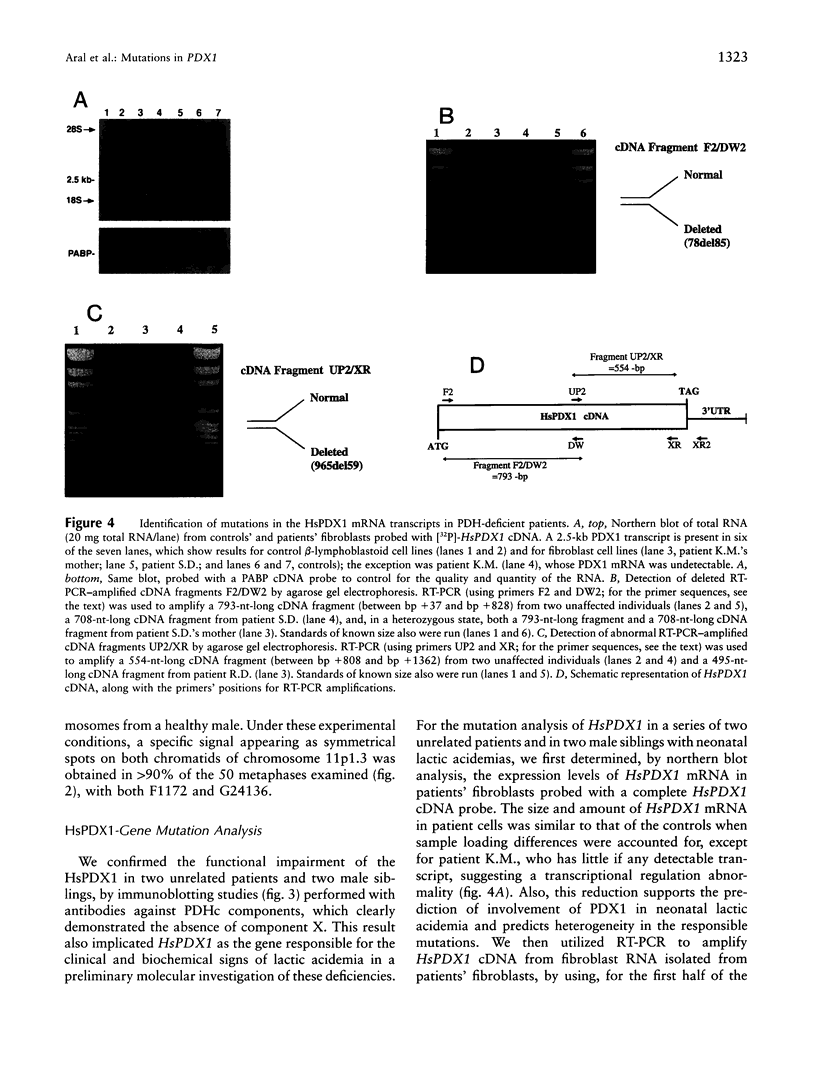
We have identified and sequenced a cDNA that encodes an apparent human orthologue of a yeast protein-X component (ScPDX1) of pyruvate dehydrogenase multienzyme complexes. The new human cDNA that has been referred to as "HsPDX1" cDNA was cloned by use of the "database cloning" strategy and had a 1,506-bp open reading frame. The amino acid sequence of the protein encoded by the cDNA was 20% identical with that encoded by the yeast PDX1 gene and 40% identical with that encoded by the lipoate acetyltransferase component of the pyruvate dehydrogenase and included a lipoyl-bearing domain that is conserved in some dehydrogenase enzyme complexes. Northern blot analysis demonstrated that the major HsPDX1 mRNA was 2.5 kb in length and was expressed mainly in human skeletal and cardiac muscles but was also present, at low levels, in other tissues. FISH analysis performed with a P1-derived artificial chromosome (PAC)-containing HsPDX1 gene sublocalized the gene to 11p1.3. Molecular investigation of PDX1 deficiency in four patients with neonatal lactic acidemias revealed mutations 78del85 and 965del59 in a homozygous state, and one other patient had no PDX1 mRNA expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aral B., Schlenzig J. S., Liu G., Kamoun P. Database cloning human delta 1-pyrroline-5-carboxylate synthetase (P5CS) cDNA: a bifunctional enzyme catalyzing the first 2 steps in proline biosynthesis. C R Acad Sci III. 1996 Mar;319(3):171–178. [PubMed] [Google Scholar]
- Bassett D. E., Jr, Boguski M. S., Spencer F., Reeves R., Goebl M., Hieter P. Comparative genomics, genome cross-referencing and XREFdb. Trends Genet. 1995 Sep;11(9):372–373. doi: 10.1016/s0168-9525(00)89109-x. [DOI] [PubMed] [Google Scholar]
- Bassett D. E., Jr, Boguski M. S., Spencer F., Reeves R., Kim S., Weaver T., Hieter P. Genome cross-referencing and XREFdb: implications for the identification and analysis of genes mutated in human disease. Nat Genet. 1997 Apr;15(4):339–344. doi: 10.1038/ng0497-339. [DOI] [PubMed] [Google Scholar]
- Behal R. H., Browning K. S., Hall T. B., Reed L. J. Cloning and nucleotide sequence of the gene for protein X from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8732–8736. doi: 10.1073/pnas.86.22.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- De Marcucci O. G., Hodgson J. A., Lindsay J. G. The Mr-50 000 polypeptide of mammalian pyruvate dehydrogenase complex participates in the acetylation reactions. Eur J Biochem. 1986 Aug 1;158(3):587–594. doi: 10.1111/j.1432-1033.1986.tb09795.x. [DOI] [PubMed] [Google Scholar]
- De Marcucci O., Lindsay J. G. Component X. An immunologically distinct polypeptide associated with mammalian pyruvate dehydrogenase multi-enzyme complex. Eur J Biochem. 1985 Jun 18;149(3):641–648. doi: 10.1111/j.1432-1033.1985.tb08972.x. [DOI] [PubMed] [Google Scholar]
- Dodt G., Braverman N., Valle D., Gould S. J. From expressed sequence tags to peroxisome biogenesis disorder genes. Ann N Y Acad Sci. 1996 Dec 27;804:516–523. doi: 10.1111/j.1749-6632.1996.tb18641.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1994 Apr 8;77(1):1–166. [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A. Rapid amplification of complementary DNA ends for generation of full-length complementary DNAs: thermal RACE. Methods Enzymol. 1993;218:340–356. doi: 10.1016/0076-6879(93)18026-9. [DOI] [PubMed] [Google Scholar]
- Geoffroy V., Fouque F., Benelli C., Poggi F., Saudubray J. M., Lissens W., Meirleir L. D., Marsac C., Lindsay J. G., Sanderson S. J. Defect in the X-lipoyl-containing component of the pyruvate dehydrogenase complex in a patient with neonatal lactic acidemia. Pediatrics. 1996 Feb;97(2):267–272. [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J. A., De Marcucci O. G., Lindsay J. G. Lipoic acid is the site of substrate-dependent acetylation of component X in ox heart pyruvate dehydrogenase multienzyme complex. Eur J Biochem. 1986 Aug 1;158(3):595–600. doi: 10.1111/j.1432-1033.1986.tb09796.x. [DOI] [PubMed] [Google Scholar]
- Jilka J. M., Rahmatullah M., Kazemi M., Roche T. E. Properties of a newly characterized protein of the bovine kidney pyruvate dehydrogenase complex. J Biol Chem. 1986 Feb 5;261(4):1858–1867. [PubMed] [Google Scholar]
- Kozak M. Regulation of translation in eukaryotic systems. Annu Rev Cell Biol. 1992;8:197–225. doi: 10.1146/annurev.cb.08.110192.001213. [DOI] [PubMed] [Google Scholar]
- Lawson J. E., Behal R. H., Reed L. J. Disruption and mutagenesis of the Saccharomyces cerevisiae PDX1 gene encoding the protein X component of the pyruvate dehydrogenase complex. Biochemistry. 1991 Mar 19;30(11):2834–2839. doi: 10.1021/bi00225a015. [DOI] [PubMed] [Google Scholar]
- Liu G., Maunoury C., Kamoun P., Aral B. Assignment of the human gene encoding the delta 1-pyrroline-5-carboxylate synthetase (P5CS) to 10q24.3 by in situ hybridization. Genomics. 1996 Oct 1;37(1):145–146. doi: 10.1006/geno.1996.0535. [DOI] [PubMed] [Google Scholar]
- Marsac C., Stansbie D., Bonne G., Cousin J., Jehenson P., Benelli C., Leroux J. P., Lindsay G. Defect in the lipoyl-bearing protein X subunit of the pyruvate dehydrogenase complex in two patients with encephalomyelopathy. J Pediatr. 1993 Dec;123(6):915–920. doi: 10.1016/s0022-3476(05)80387-7. [DOI] [PubMed] [Google Scholar]
- Neagle J. C., Lindsay J. G. Selective proteolysis of the protein X subunit of the bovine heart pyruvate dehydrogenase complex. Effects on dihydrolipoamide dehydrogenase (E3) affinity and enzymic properties of the complex. Biochem J. 1991 Sep 1;278(Pt 2):423–427. doi: 10.1042/bj2780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X. D., Browning K. S., Behal R. H., Reed L. J. Cloning and nucleotide sequence of the gene for dihydrolipoamide acetyltransferase from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7546–7550. doi: 10.1073/pnas.85.20.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers-Greenwood S. L., Rahmatullah M., Radke G. A., Roche T. E. Separation of protein X from the dihydrolipoyl transacetylase component of the mammalian pyruvate dehydrogenase complex and function of protein X. J Biol Chem. 1989 Mar 5;264(7):3655–3657. [PubMed] [Google Scholar]
- Rahmatullah M., Gopalakrishnan S., Radke G. A., Roche T. E. Domain structures of the dihydrolipoyl transacetylase and the protein X components of mammalian pyruvate dehydrogenase complex. Selective cleavage by protease Arg C. J Biol Chem. 1989 Jan 15;264(2):1245–1251. [PubMed] [Google Scholar]
- Rahmatullah M., Roche T. E. The catalytic requirements for reduction and acetylation of protein X and the related regulation of various forms of resolved pyruvate dehydrogenase kinase. J Biol Chem. 1987 Jul 25;262(21):10265–10271. [PubMed] [Google Scholar]
- Rice J. E., Dunbar B., Lindsay J. G. Sequences directing dihydrolipoamide dehydrogenase (E3) binding are located on the 2-oxoglutarate dehydrogenase (E1) component of the mammalian 2-oxoglutarate dehydrogenase multienzyme complex. EMBO J. 1992 Sep;11(9):3229–3235. doi: 10.1002/j.1460-2075.1992.tb05400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., MacKay N., Petrova-Benedict R., Ozalp I., Coskun T., Stacpoole P. W. Defects in the E2 lipoyl transacetylase and the X-lipoyl containing component of the pyruvate dehydrogenase complex in patients with lactic acidemia. J Clin Invest. 1990 Jun;85(6):1821–1824. doi: 10.1172/JCI114641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson S. J., Khan S. S., McCartney R. G., Miller C., Lindsay J. G. Reconstitution of mammalian pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes: analysis of protein X involvement and interaction of homologous and heterologous dihydrolipoamide dehydrogenases. Biochem J. 1996 Oct 1;319(Pt 1):109–116. doi: 10.1042/bj3190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thekkumkara T. J., Ho L., Wexler I. D., Pons G., Liu T. C., Patel M. S. Nucleotide sequence of a cDNA for the dihydrolipoamide acetyltransferase component of human pyruvate dehydrogenase complex. FEBS Lett. 1988 Nov 21;240(1-2):45–48. doi: 10.1016/0014-5793(88)80337-5. [DOI] [PubMed] [Google Scholar]
- Wang L. F., Voysey R., Yu M. Simplified large-scale alkaline lysis preparation of plasmid DNA with minimal use of phenol. Biotechniques. 1994 Jul;17(1):26–28. [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





