Abstract
We have studied a four-generation family with features of Weyers acrofacial dysostosis, in which the proband has a more severe phenotype, resembling Ellis-van Creveld syndrome. Weyers acrofacial dysostosis is an autosomal dominant condition with dental anomalies, nail dystrophy, postaxial polydactyly, and mild short stature. Ellis-van Creveld syndrome is a similar condition, with autosomal recessive inheritance and the additional features of disproportionate dwarfism, thoracic dysplasia, and congenital heart disease. Linkage and haplotype analysis determined that the disease locus in this pedigree resides on chromosome 4p16, distal to the genetic marker D4S3007 and within a 17-cM region flanking the genetic locus D4S2366. This region includes the Ellis-van Creveld syndrome locus, which previously was reported to map within a 3-cM region between genetic markers D4S2957 and D4S827. Either the genes for the condition in our family and for Ellis-van Creveld syndrome are near one another or these two conditions are allelic with mutations in the same gene. These data also raise the possibility that Weyers acrofacial dysostosis is the heterozygous expression of a mutation that, in homozygous form, causes the autosomal recessive disorder Ellis-van Creveld syndrome.
Full text
PDF
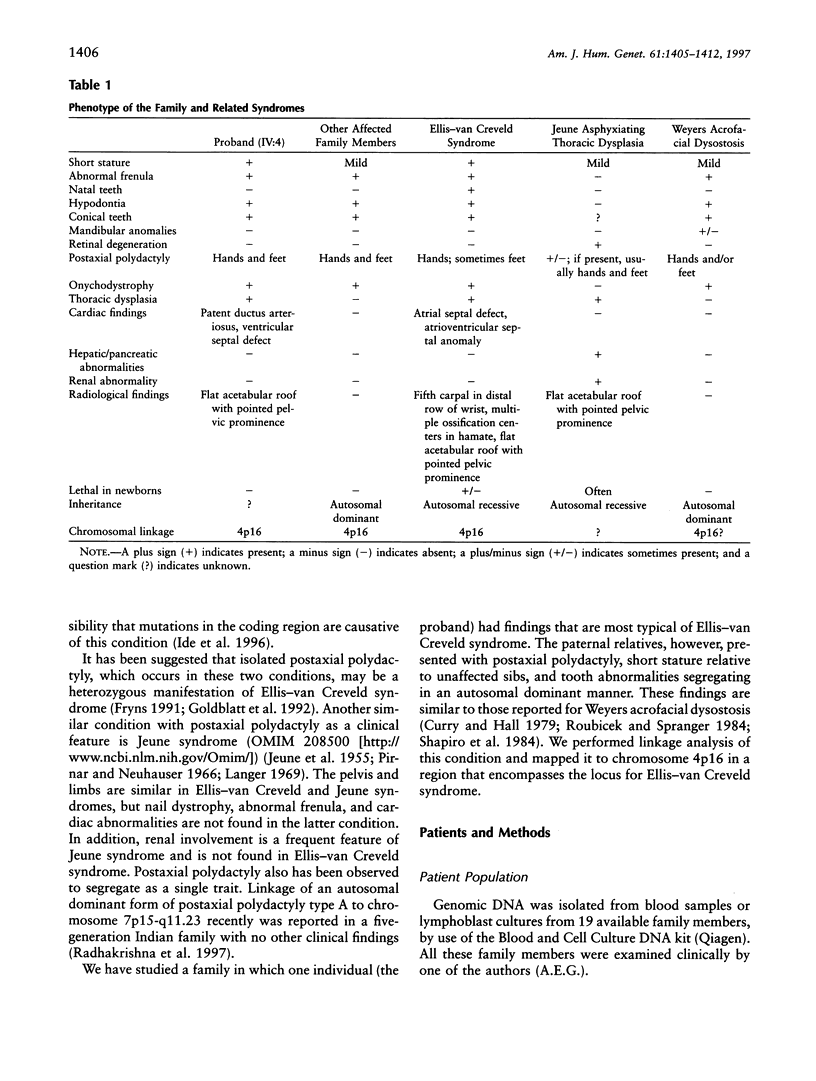

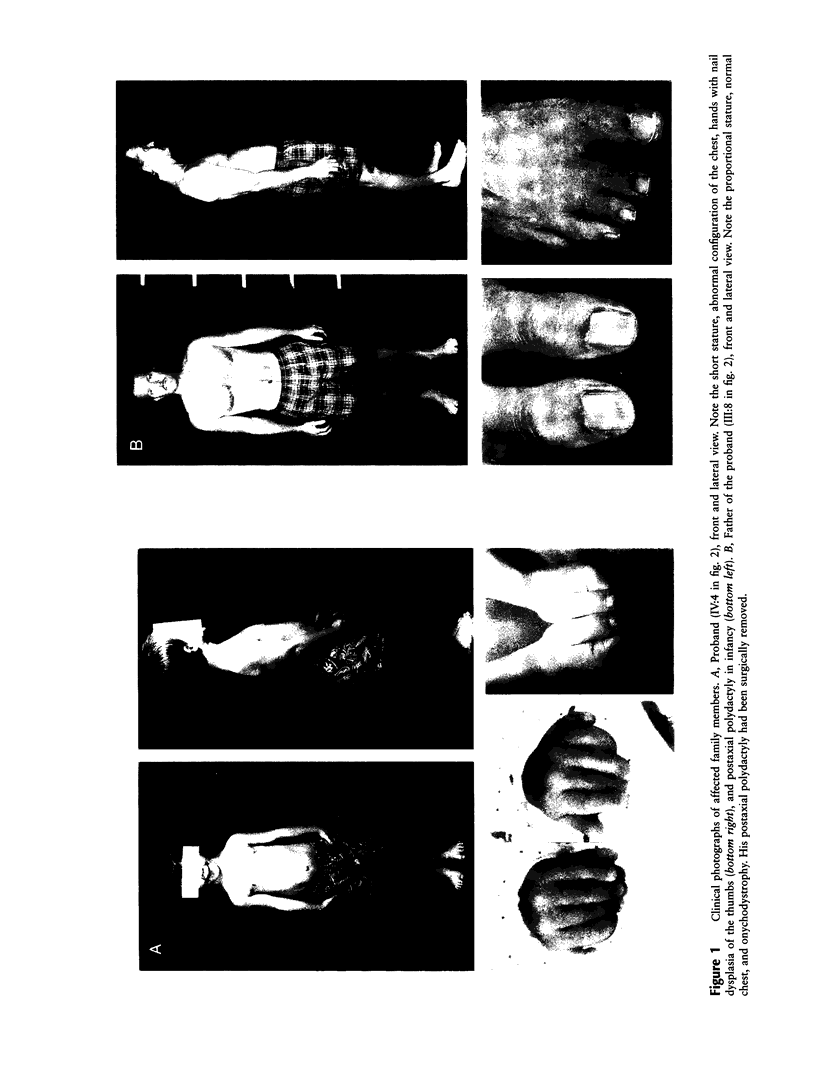
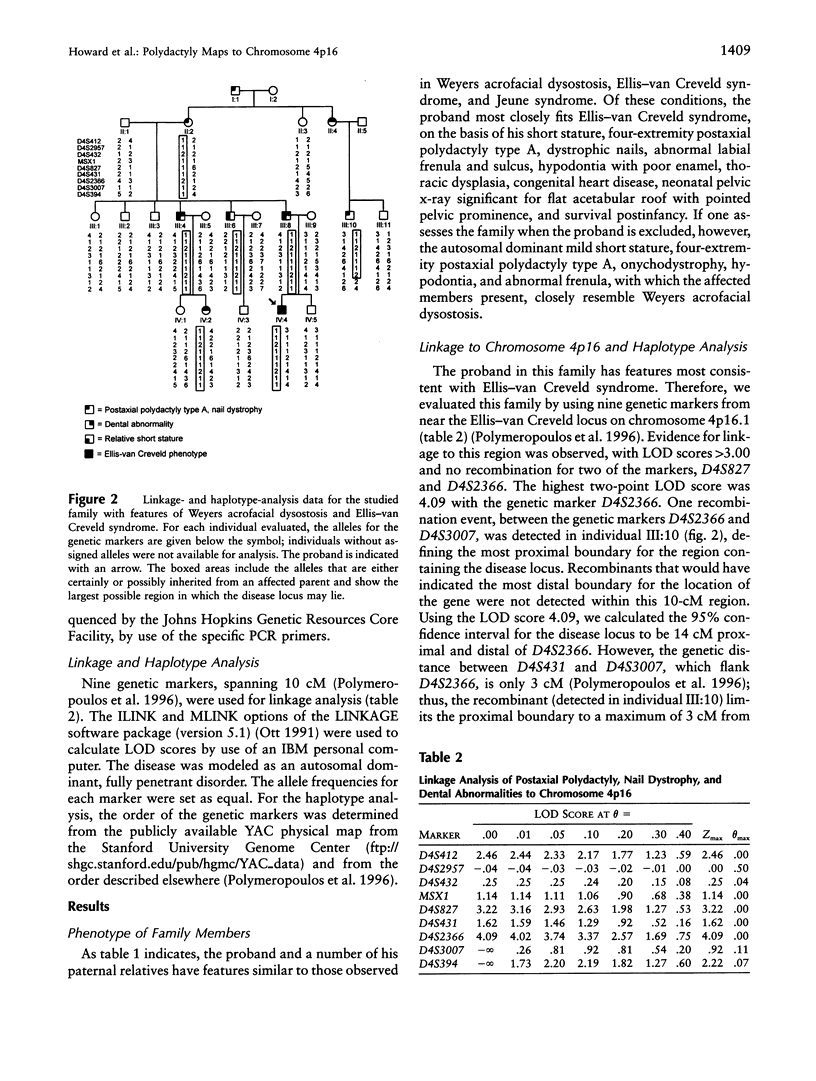



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellus G. A., Gaudenz K., Zackai E. H., Clarke L. A., Szabo J., Francomano C. A., Muenke M. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nat Genet. 1996 Oct;14(2):174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- Bellus G. A., McIntosh I., Smith E. A., Aylsworth A. S., Kaitila I., Horton W. A., Greenhaw G. A., Hecht J. T., Francomano C. A. A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet. 1995 Jul;10(3):357–359. doi: 10.1038/ng0795-357. [DOI] [PubMed] [Google Scholar]
- Biggerstaff R. H., Mazaheri M. Oral manifestations of the Ellis-van Creveld syndrome. J Am Dent Assoc. 1968 Nov;77(5):1090–1095. doi: 10.14219/jada.archive.1968.0342. [DOI] [PubMed] [Google Scholar]
- Christiano A. M., Greenspan D. S., Hoffman G. G., Zhang X., Tamai Y., Lin A. N., Dietz H. C., Hovnanian A., Uitto J. A missense mutation in type VII collagen in two affected siblings with recessive dystrophic epidermolysis bullosa. Nat Genet. 1993 May;4(1):62–66. doi: 10.1038/ng0593-62. [DOI] [PubMed] [Google Scholar]
- Christiano A. M., Ryynänen M., Uitto J. Dominant dystrophic epidermolysis bullosa: identification of a Gly-->Ser substitution in the triple-helical domain of type VII collagen. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3549–3553. doi: 10.1073/pnas.91.9.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry C. J., Hall B. D. Polydactyly, conical teeth, nail dysplasia, and short limbs: a new autosomal dominant malformation syndrome. Birth Defects Orig Artic Ser. 1979;15(5B):253–263. [PubMed] [Google Scholar]
- De Paepe A., Nuytinck L., Raes M., Fryns J. P. Homozygosity by descent for a COL1A2 mutation in two sibs with severe osteogenesis imperfecta and mild clinical expression in the heterozygotes. Hum Genet. 1997 Apr;99(4):478–483. doi: 10.1007/s004390050392. [DOI] [PubMed] [Google Scholar]
- DeBry R. W., Seldin M. F. Human/mouse homology relationships. Genomics. 1996 May 1;33(3):337–351. doi: 10.1006/geno.1996.0209. [DOI] [PubMed] [Google Scholar]
- Fryns J. P. Postaxial polydactyly as heterozygote manifestation in Ellis-Van Creveld syndrome? Am J Med Genet. 1991 Jun 15;39(4):500–501. doi: 10.1002/ajmg.1320390430. [DOI] [PubMed] [Google Scholar]
- Goldblatt J., Minutillo C., Pemberton P. J., Hurst J. Ellis-van Creveld syndrome in a Western Australian aboriginal community. Postaxial polydactyly as a heterozygous manifestation? Med J Aust. 1992 Aug 17;157(4):271–272. [PubMed] [Google Scholar]
- Hayashi Y., Sunthornthepvarakul T., Refetoff S. Mutations of CpG dinucleotides located in the triiodothyronine (T3)-binding domain of the thyroid hormone receptor (TR) beta gene that appears to be devoid of natural mutations may not be detected because they are unlikely to produce the clinical phenotype of resistance to thyroid hormone. J Clin Invest. 1994 Aug;94(2):607–615. doi: 10.1172/JCI117376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. E., Clark L. N., Ivens A., Williamson R. Structure and sequence of the human homeobox gene HOX7. Genomics. 1991 Nov;11(3):670–678. doi: 10.1016/0888-7543(91)90074-o. [DOI] [PubMed] [Google Scholar]
- Ide S. E., Ortiz de Luna R. I., Francomano C. A., Polymeropoulos M. H. Exclusion of the MSX1 homeobox gene as the gene for the Ellis van Creveld syndrome in the Amish. Hum Genet. 1996 Nov;98(5):572–575. doi: 10.1007/s004390050261. [DOI] [PubMed] [Google Scholar]
- MCKUSICK V. A., EGELAND J. A., ELDRIDGE R., KRUSEN D. E. DWARFISM IN THE AMISH I. THE ELLIS-VAN CREVELD SYNDROME. Bull Johns Hopkins Hosp. 1964 Oct;115:306–336. [PubMed] [Google Scholar]
- MacKenzie A., Purdie L., Davidson D., Collinson M., Hill R. E. Two enhancer domains control early aspects of the complex expression pattern of Msx1. Mech Dev. 1997 Feb;62(1):29–40. doi: 10.1016/s0925-4773(96)00646-6. [DOI] [PubMed] [Google Scholar]
- Meyers G. A., Orlow S. J., Munro I. R., Przylepa K. A., Jabs E. W. Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in Crouzon syndrome with acanthosis nigricans. Nat Genet. 1995 Dec;11(4):462–464. doi: 10.1038/ng1295-462. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M. H., Ide S. E., Wright M., Goodship J., Weissenbach J., Pyeritz R. E., Da Silva E. O., Ortiz De Luna R. I., Francomano C. A. The gene for the Ellis-van Creveld syndrome is located on chromosome 4p16. Genomics. 1996 Jul 1;35(1):1–5. doi: 10.1006/geno.1996.0315. [DOI] [PubMed] [Google Scholar]
- Pruchno C. J., Cohn D. H., Wallis G. A., Willing M. C., Starman B. J., Zhang X. M., Byers P. H. Osteogenesis imperfecta due to recurrent point mutations at CpG dinucleotides in the COL1A1 gene of type I collagen. Hum Genet. 1991 May;87(1):33–40. doi: 10.1007/BF01213088. [DOI] [PubMed] [Google Scholar]
- Radhakrishna U., Blouin J. L., Mehenni H., Patel U. C., Patel M. N., Solanki J. V., Antonarakis S. E. Mapping one form of autosomal dominant postaxial polydactyly type A to chromosome 7p15-q11.23 by linkage analysis. Am J Hum Genet. 1997 Mar;60(3):597–604. [PMC free article] [PubMed] [Google Scholar]
- Robert B., Sassoon D., Jacq B., Gehring W., Buckingham M. Hox-7, a mouse homeobox gene with a novel pattern of expression during embryogenesis. EMBO J. 1989 Jan;8(1):91–100. doi: 10.1002/j.1460-2075.1989.tb03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P. J., Cowley G. S., McGee T. L., Sandberg M. A., Berson E. L., Dryja T. P. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nat Genet. 1992 Jun;1(3):209–213. doi: 10.1038/ng0692-209. [DOI] [PubMed] [Google Scholar]
- Roubicek M., Spranger J. Weyers acrodental dysostosis in a family. Clin Genet. 1984 Dec;26(6):587–590. doi: 10.1111/j.1399-0004.1984.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Bonaventure J., Legeai-Mallet L., Pelet A., Rozet J. M., Maroteaux P., Le Merrer M., Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994 Sep 15;371(6494):252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Rousseau F., el Ghouzzi V., Delezoide A. L., Legeai-Mallet L., Le Merrer M., Munnich A., Bonaventure J. Missense FGFR3 mutations create cysteine residues in thanatophoric dwarfism type I (TD1). Hum Mol Genet. 1996 Apr;5(4):509–512. doi: 10.1093/hmg/5.4.509. [DOI] [PubMed] [Google Scholar]
- Shapiro S. D., Jorgenson R. J., Salinas C. F. Brief clinical report: Curry-Hall syndrome. Am J Med Genet. 1984 Mar;17(3):579–583. doi: 10.1002/ajmg.1320170305. [DOI] [PubMed] [Google Scholar]
- Shiang R., Thompson L. M., Zhu Y. Z., Church D. M., Fielder T. J., Bocian M., Winokur S. T., Wasmuth J. J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994 Jul 29;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Spranger S., Tariverdian G. Symptomatic heterozygosity in the Ellis-van Creveld syndrome? Clin Genet. 1995 Apr;47(4):217–220. doi: 10.1111/j.1399-0004.1995.tb03963.x. [DOI] [PubMed] [Google Scholar]
- Takeda K., Sakurai A., DeGroot L. J., Refetoff S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor-beta gene. J Clin Endocrinol Metab. 1992 Jan;74(1):49–55. doi: 10.1210/jcem.74.1.1727829. [DOI] [PubMed] [Google Scholar]
- Tavormina P. L., Shiang R., Thompson L. M., Zhu Y. Z., Wilkin D. J., Lachman R. S., Wilcox W. R., Rimoin D. L., Cohn D. H., Wasmuth J. J. Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet. 1995 Mar;9(3):321–328. doi: 10.1038/ng0395-321. [DOI] [PubMed] [Google Scholar]
- Taylor G. A., Jordan C. E., Dorst S. K., Dorst J. P. Polycarpaly and other abnormalities of the wrist in chondroectodermal dysplasia: the Ellis-van Creveld syndrome. Radiology. 1984 May;151(2):393–396. doi: 10.1148/radiology.151.2.6709909. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Hesketh C., Taylor P., Temperley I. J., Hutchinson R. M., Old J. M., Wood W. G., Clegg J. B., Weatherall D. J. Molecular basis for dominantly inherited inclusion body beta-thalassemia. Proc Natl Acad Sci U S A. 1990 May;87(10):3924–3928. doi: 10.1073/pnas.87.10.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. M., Plummer S., Schalling M., Altherr M. R., Gusella J. F., Housman D. E., Wasmuth J. J. A gene encoding a fibroblast growth factor receptor isolated from the Huntington disease gene region of human chromosome 4. Genomics. 1991 Dec;11(4):1133–1142. doi: 10.1016/0888-7543(91)90041-c. [DOI] [PubMed] [Google Scholar]
- Vastardis H., Karimbux N., Guthua S. W., Seidman J. G., Seidman C. E. A human MSX1 homeodomain missense mutation causes selective tooth agenesis. Nat Genet. 1996 Aug;13(4):417–421. doi: 10.1038/ng0896-417. [DOI] [PubMed] [Google Scholar]
- WEYERS H. Uber eine korrelierte Missbildung der Kiefer- und Extremitätenakren. Fortschr Geb Rontgenstr. 1952 Nov;77(5):562–567. [PubMed] [Google Scholar]
- Zimmerman T. S., Ruggeri Z. M. von Willebrand disease. Hum Pathol. 1987 Feb;18(2):140–152. doi: 10.1016/s0046-8177(87)80332-5. [DOI] [PubMed] [Google Scholar]