Abstract
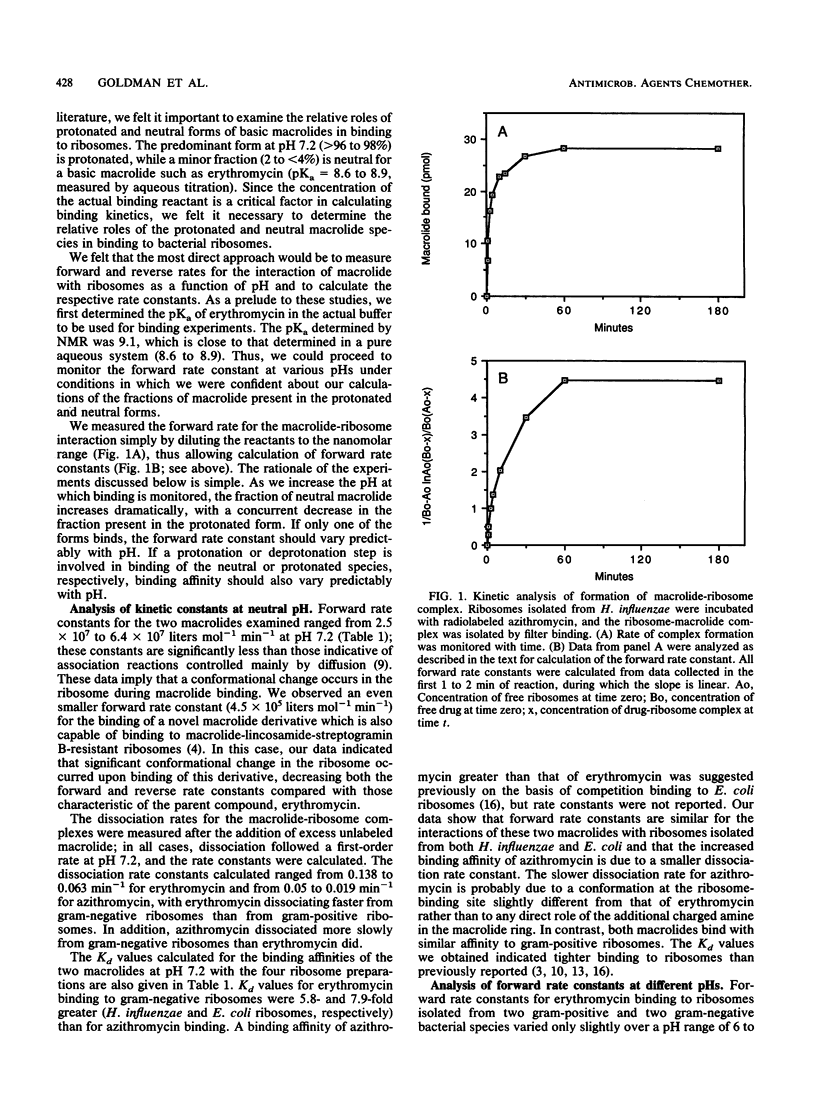
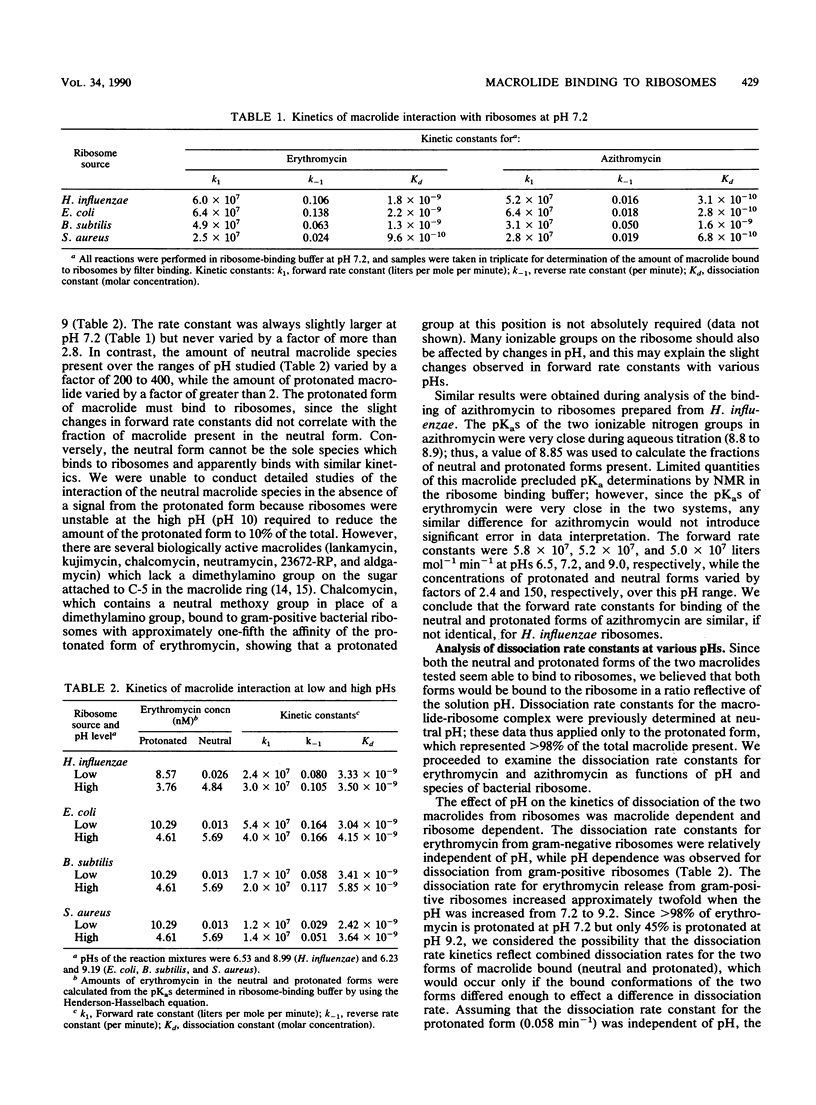
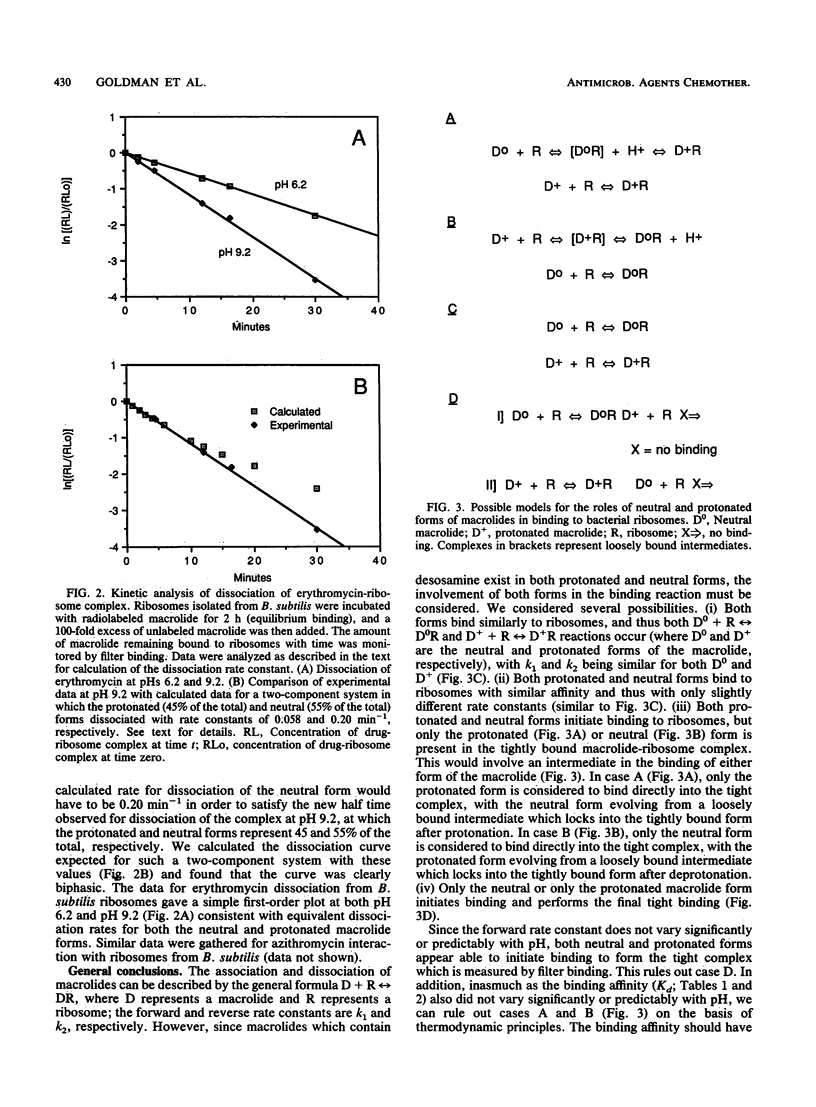
Erythromycin binds to a single site on the bacterial 50S ribosomal subunit and perturbs protein synthesis. However, erythromycin contains desosamine and thus exists in both protonated (greater than 96%) and neutral (less than 4%) forms at physiological pH because of the pKa of the dimethylamino group. We therefore examined the relative roles of both forms in binding to ribosomes isolated from two species each of gram-positive and gram-negative bacteria. We developed a system to directly measure the forward (association) rate constant of formation of the macrolide-ribosome complex, and we have measured both the forward and reverse (dissociation) rate constants as a function of pH. Forward rate constants and binding affinity did not correlate with pH when the interaction of erythromycin with ribosomes from both gram-positive and gram-negative bacteria was examined, demonstrating that the protonated form of this macrolide binds to ribosomes. Conversely, the neutral form of macrolide cannot be the sole binding species and appears to bind with the same kinetics as the protonated form. Forward rate constants were 3- to 4-fold greater at physiological pH, and binding affinity calculated from rate constants was 5- to 10-fold greater than previously estimated. Similar results were obtained with azithromycin, a novel 15-membered macrolide that contains an additional tertiary amine in the macrolide ring. Ribosome- and macrolide-specific kinetic parameters were demonstrated at neutral pH and may be related to the potency of the two macrolides against gram-positive and gram-negative bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dette G. A., Knothe H., Kaula S. Modifying effects of pH and temperature on (14C)erythromycin uptake into Staphylococcus aureus--relation to antimicrobial activity. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jul;265(3-4):393–403. [PubMed] [Google Scholar]
- Di Giambattista M., Engelborghs Y., Nyssen E., Cocito C. Kinetics of binding of macrolides, lincosamides, and synergimycins to ribosomes. J Biol Chem. 1987 Jun 25;262(18):8591–8597. [PubMed] [Google Scholar]
- Goldman R. C., Kadam S. K. Binding of novel macrolide structures to macrolides-lincosamides-streptogramin B-resistant ribosomes inhibits protein synthesis and bacterial growth. Antimicrob Agents Chemother. 1989 Jul;33(7):1058–1066. doi: 10.1128/aac.33.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Knowles D. J. Uptake of erythromycin by McCoy and HEp2 cells: its dependence on cellular pH gradients. J Antimicrob Chemother. 1988 Jun;21(6):765–772. doi: 10.1093/jac/21.6.765. [DOI] [PubMed] [Google Scholar]
- Langlois R., Cantor C. R., Vince R., Pestka S. Interaction between the erythromycin and chloramphenicol binding sites on the Escherichica coli ribosome. Biochemistry. 1977 May 31;16(11):2349–2356. doi: 10.1021/bi00630a007. [DOI] [PubMed] [Google Scholar]
- Langlois R., Lee C. C., Cantor C. R., Vince R., Pestka S. The distance between two functionally significant regions of the 50 S Escherichia coli ribosome: the erythromycin binding site and proteins L7/L12. J Mol Biol. 1976 Sep 15;106(2):297–313. doi: 10.1016/0022-2836(76)90087-5. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N. A survey of x-ray diffraction studies of enzyme-other molecule interactions as possible models for receptor sites. Ann N Y Acad Sci. 1981;367:326–339. doi: 10.1111/j.1749-6632.1981.tb50576.x. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. The intermolecular complex of erythromycin and ribosome. J Mol Biol. 1969 Sep 14;44(2):347–361. doi: 10.1016/0022-2836(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Mao J. C. The stoichiometry of erythromycin binding to ribosomal particles of Staphylococcus aureus. Biochem Pharmacol. 1967 Dec;16(12):2441–2443. doi: 10.1016/0006-2952(67)90232-8. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Wiegand R. G. Mode of action of macrolides. Biochim Biophys Acta. 1968 Apr 22;157(2):404–413. doi: 10.1016/0005-2787(68)90094-4. [DOI] [PubMed] [Google Scholar]
- Oleinick N. L., Corcoran J. W. Two types of binding of erythromycin to ribosomes from antibiotic-sensitive and -resistant Bacillus subtilis 168. J Biol Chem. 1969 Feb 25;244(4):727–735. [PubMed] [Google Scholar]
- Omura S., Namiki S., Shibata M., Muro T., Sawada J. Studies on the antibiotics from Streptomyces spinichromogenes var. kujimyceticus. V. Some antimicrobial characteristics of kujimycin A and kujimycin B against macrolide resistant staphylococci. J Antibiot (Tokyo) 1970 Sep;23(9):448–460. doi: 10.7164/antibiotics.23.448. [DOI] [PubMed] [Google Scholar]
- Pestka S. Binding of [14C]erythromycin to Escherichia coli ribosomes. Antimicrob Agents Chemother. 1974 Oct;6(4):474–478. doi: 10.1128/aac.6.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Lorian V., Gerstein D., Loder P. B., Finland M. Enhancing effect on alkalinization of the medium on the activity of erythromycin against gram-negative bacteria. Appl Microbiol. 1968 Sep;16(9):1288–1292. doi: 10.1128/am.16.9.1288-1292.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman S. B., Jones N. R., Young F. E., Corcoran J. W. Sensitivity and resistance to erythromycin in Bacillus subtilis 168: the ribosomal binding of erythromycin and chloramphenicol. Biochim Biophys Acta. 1966 Aug 17;123(2):438–440. doi: 10.1016/0005-2787(66)90301-7. [DOI] [PubMed] [Google Scholar]
- Yunker M. H., Borodkin S. Determination of rate constants for in vitro three-compartment transfer using a two-phase system. J Pharm Sci. 1971 Jan;60(1):52–56. doi: 10.1002/jps.2600600108. [DOI] [PubMed] [Google Scholar]



