Abstract
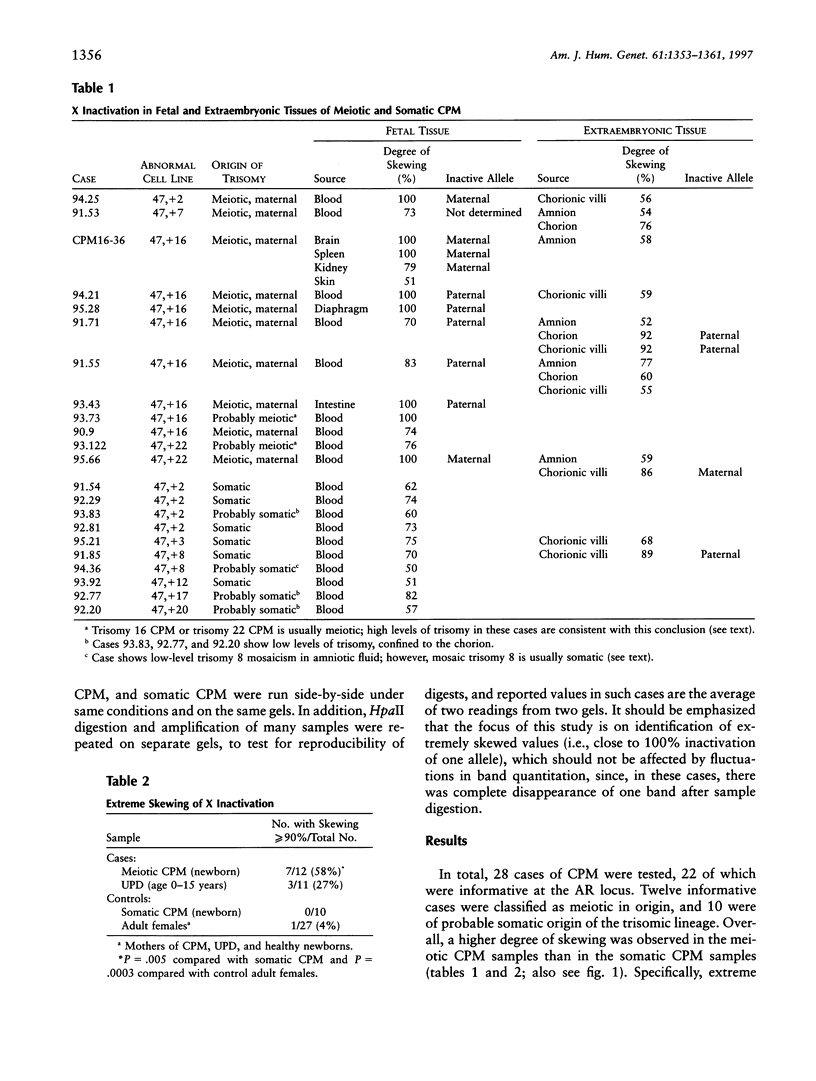
The inactivation of one X chromosome in females is normally random with regard to which X is inactivated. However, exclusive or almost-exclusive inactivation of one X may be observed in association with some X-autosomal rearrangements, mutations of the XIST gene, certain X-linked diseases, and MZ twinning. In the present study, a methylation difference near a polymorphism in the X-linked androgen-receptor gene was used to investigate the possibility that nonrandom X inactivation is increases in fetuses and newborns that are associated with confined placental mosaicism (CPM) involving an autosomal trisomy. Extreme skewing was observed in 7 (58%) of 12 cases with a meiotic origin of the trisomy, but in none of 10 cases examined with a somatic origin of the trisomy, and in only 1 (4%) of 27 control adult females. In addition, an extremely skewed X-inactivation pattern was observed in 3 of 10 informative cases of female uniparental disomy (UPD) of chromosome 15. This may reflect the fact that a proportion of UPD cases arise by "rescue" of a chromosomally abnormal conceptus and are therefore associated with CPM. A skewed pattern of X inactivation in CPM cases is hypothesized to result from a reduction in the size of the early-embryonic cell pool, because of either poor early growth or subsequent selection against the trisomic cells. Since approximately 2% of pregnancies detected by chorionic villus sampling are associated with CPM, this is likely a significant contributor to both skewed X inactivation observed in the newborn population and the expression of recessive X-linked diseases in females.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Belmont J. W. Genetic control of X inactivation and processes leading to X-inactivation skewing. Am J Hum Genet. 1996 Jun;58(6):1101–1108. [PMC free article] [PubMed] [Google Scholar]
- Bogart M. H., Miyabara S. The production of mouse fetal-placental chimeras using trisomy 16 and euploid blastocysts. Anat Embryol (Berl) 1990;181(2):137–147. doi: 10.1007/BF00198953. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Robinson W. P. XIST expression and X-chromosome inactivation in human preimplantation embryos. Am J Hum Genet. 1997 Jul;61(1):5–8. doi: 10.1086/513914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busque L., Mio R., Mattioli J., Brais E., Blais N., Lalonde Y., Maragh M., Gilliland D. G. Nonrandom X-inactivation patterns in normal females: lyonization ratios vary with age. Blood. 1996 Jul 1;88(1):59–65. [PubMed] [Google Scholar]
- Cassidy S. B., Lai L. W., Erickson R. P., Magnuson L., Thomas E., Gendron R., Herrmann J. Trisomy 15 with loss of the paternal 15 as a cause of Prader-Willi syndrome due to maternal disomy. Am J Hum Genet. 1992 Oct;51(4):701–708. [PMC free article] [PubMed] [Google Scholar]
- Everett C. A., West J. D. The influence of ploidy on the distribution of cells in chimaeric mouse blastocysts. Zygote. 1996 Feb;4(1):59–66. doi: 10.1017/s0967199400002896. [DOI] [PubMed] [Google Scholar]
- Gardner R. L., Lyon M. F. X chromosome inactivation studied by injection of a single cell into the mouse blastocyst. Nature. 1971 Jun 11;231(5302):385–386. doi: 10.1038/231385a0. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Goodship J., Carter J., Burn J. X-inactivation patterns in monozygotic and dizygotic female twins. Am J Med Genet. 1996 Jan 22;61(3):205–208. doi: 10.1002/(SICI)1096-8628(19960122)61:3<205::AID-AJMG3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Goto J., Figlewicz D. A., Marineau C., Khodr N., Rouleau G. A. Dinucleotide repeat polymorphism at the IGF2R locus. Nucleic Acids Res. 1992 Feb 25;20(4):923–923. doi: 10.1093/nar/20.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp A., Winking H., Herbst E. W., Claussen C. P. Murine trisomy: developmental profiles of the embryo, and isolation of trisomic cellular systems. J Exp Zool. 1983 Nov;228(2):253–269. doi: 10.1002/jez.1402280210. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Twins and twinning. Am J Med Genet. 1996 Jan 22;61(3):202–204. doi: 10.1002/(SICI)1096-8628(19960122)61:3<202::AID-AJMG2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Harrison K. B. X-chromosome inactivation in the human cytotrophoblast. Cytogenet Cell Genet. 1989;52(1-2):37–41. doi: 10.1159/000132835. [DOI] [PubMed] [Google Scholar]
- Harrison K. J., Barrett I. J., Lomax B. L., Kuchinka B. D., Kalousek D. K. Detection of confined placental mosaicism in trisomy 18 conceptions using interphase cytogenetic analysis. Hum Genet. 1993 Oct;92(4):353–358. doi: 10.1007/BF01247334. [DOI] [PubMed] [Google Scholar]
- Henderson K. G., Shaw T. E., Barrett I. J., Telenius A. H., Wilson R. D., Kalousek D. K. Distribution of mosaicism in human placentae. Hum Genet. 1996 May;97(5):650–654. doi: 10.1007/BF02281877. [DOI] [PubMed] [Google Scholar]
- James R. M., Klerkx A. H., Keighren M., Flockhart J. H., West J. D. Restricted distribution of tetraploid cells in mouse tetraploid<==>diploid chimaeras. Dev Biol. 1995 Jan;167(1):213–226. doi: 10.1006/dbio.1995.1018. [DOI] [PubMed] [Google Scholar]
- Kalousek D. K., Dill F. J. Chromosomal mosaicism confined to the placenta in human conceptions. Science. 1983 Aug 12;221(4611):665–667. doi: 10.1126/science.6867735. [DOI] [PubMed] [Google Scholar]
- Kalousek D. K., Howard-Peebles P. N., Olson S. B., Barrett I. J., Dorfmann A., Black S. H., Schulman J. D., Wilson R. D. Confirmation of CVS mosaicism in term placentae and high frequency of intrauterine growth retardation association with confined placental mosaicism. Prenat Diagn. 1991 Oct;11(10):743–750. doi: 10.1002/pd.1970111002. [DOI] [PubMed] [Google Scholar]
- Kalousek D. K., Langlois S., Barrett I., Yam I., Wilson D. R., Howard-Peebles P. N., Johnson M. P., Giorgiutti E. Uniparental disomy for chromosome 16 in humans. Am J Hum Genet. 1993 Jan;52(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- Kalousek D. K. The effect of confined placental mosaicism on development of the human aneuploid conceptus. Birth Defects Orig Artic Ser. 1993;29(1):39–51. [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H., Zachary J. M., Simpson J. L., Golbus M. S., Pergament E., Jackson L., Mahoney M. J., Desnick R. J., Schulman J., Copeland K. L. Cytogenetic results from the U.S. Collaborative Study on CVS. Prenat Diagn. 1992 May;12(5):317–345. doi: 10.1002/pd.1970120503. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Jeppesen P., Torchia B. S., Fu S., Dunn M. A., Axelman J., Schmeckpeper B. J., Fantes J., Zori R. T., Driscoll D. J. Lack of X inactivation associated with maternal X isodisomy: evidence for a counting mechanism prior to X inactivation during human embryogenesis. Am J Hum Genet. 1996 Jan;58(1):161–170. [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Moser H. W., Moser A. B., Axelman J., Sillence D., Norum R. A. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5066–5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Axelman J., Kaslow D. C., Schmidt M. Incomplete X chromosome dosage compensation in chorionic villi of human placenta. Proc Natl Acad Sci U S A. 1985 May;82(10):3390–3394. doi: 10.1073/pnas.82.10.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T. K., Passage M. B., Williams J. W., 3rd, Sparkes R. S., Yen P. H., Shapiro L. J. X-chromosome inactivation in cultured cells from human chorionic villi. Somat Cell Mol Genet. 1989 Mar;15(2):131–136. doi: 10.1007/BF01535073. [DOI] [PubMed] [Google Scholar]
- Naumova A. K., Plenge R. M., Bird L. M., Leppert M., Morgan K., Willard H. F., Sapienza C. Heritability of X chromosome--inactivation phenotype in a large family. Am J Hum Genet. 1996 Jun;58(6):1111–1119. [PMC free article] [PubMed] [Google Scholar]
- Nesbit M. N. X chromosome inactivation mosaicism in the mouse. Dev Biol. 1971 Oct;26(2):252–263. doi: 10.1016/0012-1606(71)90125-4. [DOI] [PubMed] [Google Scholar]
- Penny G. D., Kay G. F., Sheardown S. A., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996 Jan 11;379(6561):131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Plenge R. M., Hendrich B. D., Schwartz C., Arena J. F., Naumova A., Sapienza C., Winter R. M., Willard H. F. A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nat Genet. 1997 Nov;17(3):353–356. doi: 10.1038/ng1197-353. [DOI] [PubMed] [Google Scholar]
- Purvis-Smith S. G., Saville T., Manass S., Yip M. Y., Lam-Po-Tang P. R., Duffy B., Johnston H., Leigh D., McDonald B. Uniparental disomy 15 resulting from "correction" of an initial trisomy 15. Am J Hum Genet. 1992 Jun;50(6):1348–1350. [PMC free article] [PubMed] [Google Scholar]
- Robinson W. P., Barrett I. J., Bernard L., Telenius A., Bernasconi F., Wilson R. D., Best R. G., Howard-Peebles P. N., Langlois S., Kalousek D. K. Meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal intrauterine growth restriction. Am J Hum Genet. 1997 Apr;60(4):917–927. [PMC free article] [PubMed] [Google Scholar]
- Robinson W. P., Bernasconi F., Mutirangura A., Ledbetter D. H., Langlois S., Malcolm S., Morris M. A., Schinzel A. A. Nondisjunction of chromosome 15: origin and recombination. Am J Hum Genet. 1993 Sep;53(3):740–751. [PMC free article] [PubMed] [Google Scholar]
- Robinson W. P., Bottani A., Xie Y. G., Balakrishman J., Binkert F., Mächler M., Prader A., Schinzel A. Molecular, cytogenetic, and clinical investigations of Prader-Willi syndrome patients. Am J Hum Genet. 1991 Dec;49(6):1219–1234. [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Du Sart D. Functional disomies of the X chromosome influence the cell selection and hence the X inactivation pattern in females with balanced X-autosome translocations: a review of 122 cases. Am J Med Genet. 1992 Jan 15;42(2):161–169. doi: 10.1002/ajmg.1320420205. [DOI] [PubMed] [Google Scholar]
- Snow M. H. Restorative growth and its problems for morphogenesis. Prog Clin Biol Res. 1985;163C:295–299. [PubMed] [Google Scholar]
- Tan S. S., Williams E. A., Tam P. P. X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat Genet. 1993 Feb;3(2):170–174. doi: 10.1038/ng0293-170. [DOI] [PubMed] [Google Scholar]
- Wang B. B., Rubin C. H., Williams J., 3rd Mosaicism in chorionic villus sampling: an analysis of incidence and chromosomes involved in 2612 consecutive cases. Prenat Diagn. 1993 Mar;13(3):179–190. doi: 10.1002/pd.1970130305. [DOI] [PubMed] [Google Scholar]
- Zabel B. U., Baumann W. A., Pirntke W., Gerhard-Ratschow K. X-inactivation pattern in three cases of X/autosome translocation. Am J Med Genet. 1978;1(3):309–317. doi: 10.1002/ajmg.1320010307. [DOI] [PubMed] [Google Scholar]



