Abstract
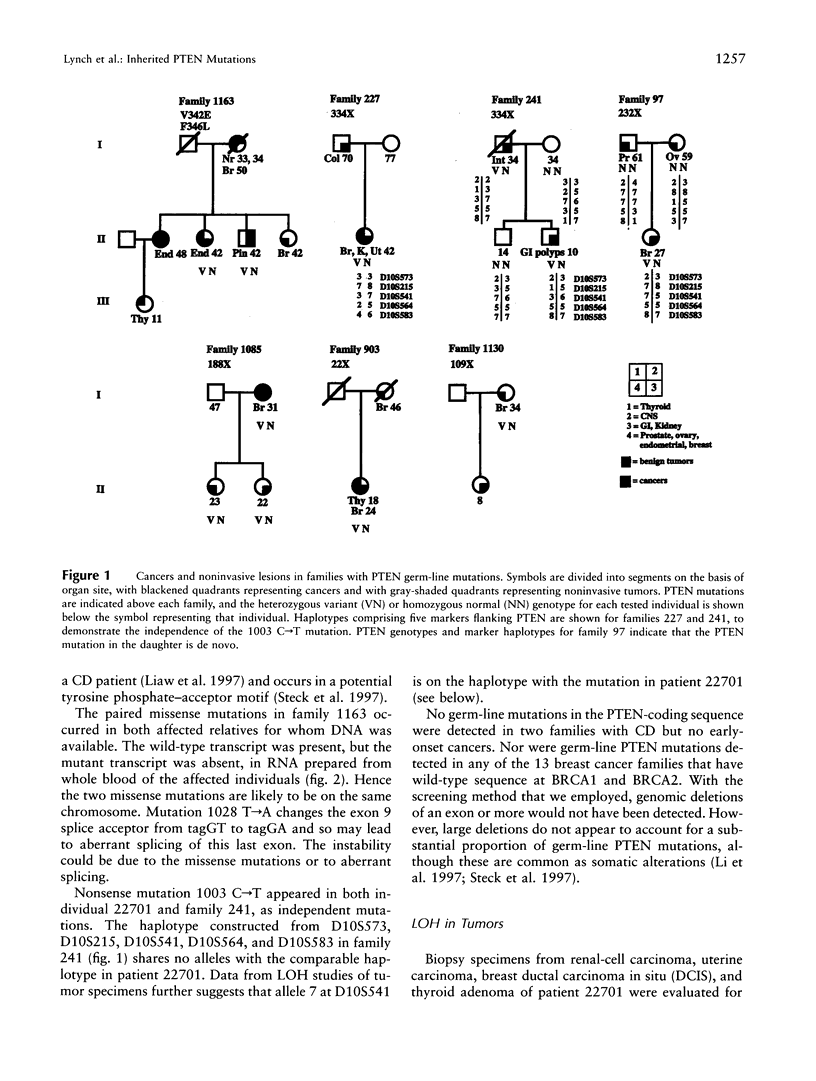
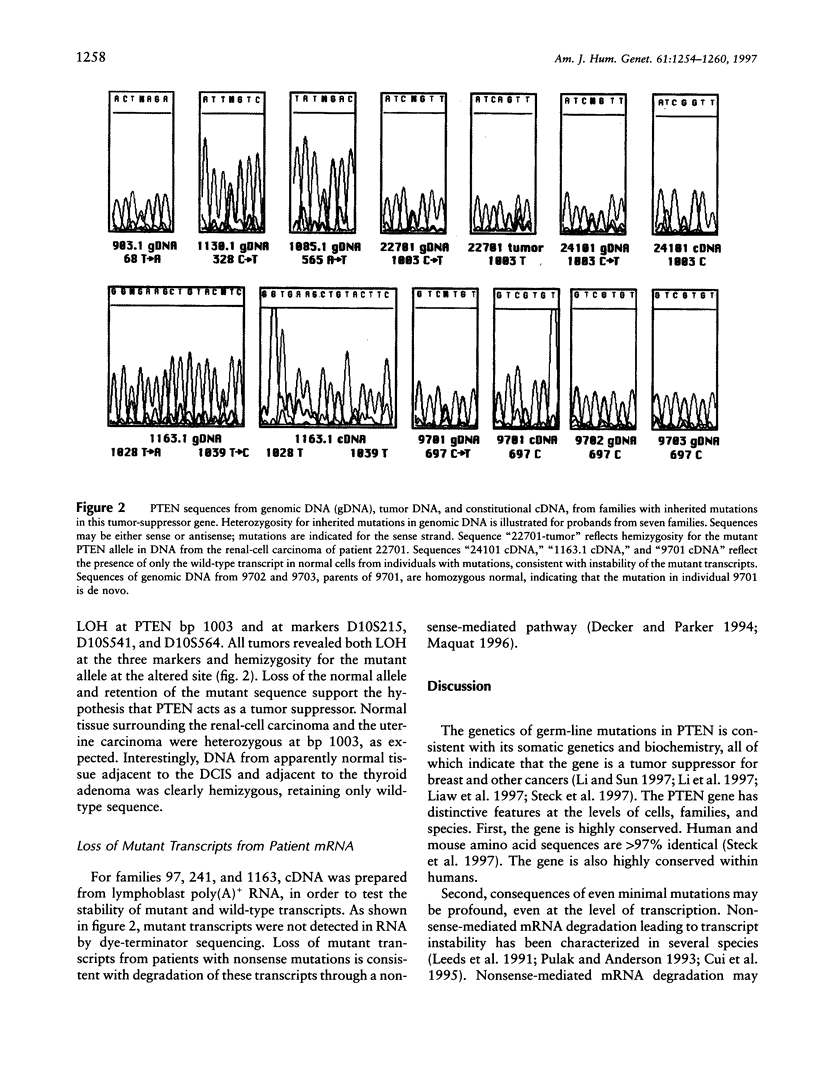
PTEN, a protein tyrosine phosphatase with homology to tensin, is a tumor-suppressor gene on chromosome 10q23. Somatic mutations in PTEN occur in multiple tumors, most markedly glioblastomas. Germ-line mutations in PTEN are responsible for Cowden disease (CD), a rare autosomal dominant multiple-hamartoma syndrome. PTEN was sequenced from constitutional DNA from 25 families. Germ-line PTEN mutations were detected in all of five families with both breast cancer and CD, in one family with juvenile polyposis syndrome, and in one of four families with breast and thyroid tumors. In this last case, signs of CD were subtle and were diagnosed only in the context of mutation analysis. PTEN mutations were not detected in 13 families at high risk of breast and/or ovarian cancer. No PTEN-coding-sequence polymorphisms were detected in 70 independent chromosomes. Seven PTEN germ-line mutations occurred, five nonsense and two missense mutations, in six of nine PTEN exons. The wild-type PTEN allele was lost from renal, uterine, breast, and thyroid tumors from a single patient. Loss of PTEN expression was an early event, reflected in loss of the wild-type allele in DNA from normal tissue adjacent to the breast and thyroid tumors. In RNA from normal tissues from three families, mutant transcripts appeared unstable. Germ-line PTEN mutations predispose to breast cancer in association with CD, although the signs of CD may be subtle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S., Haber R. M., Goodman J. C., Duvic M. Cowden syndrome and Lhermitte-Duclos disease. Cancer. 1992 Aug 15;70(4):869–876. doi: 10.1002/1097-0142(19920815)70:4<869::aid-cncr2820700424>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cui Y., Hagan K. W., Zhang S., Peltz S. W. Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon. Genes Dev. 1995 Feb 15;9(4):423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- Decker C. J., Parker R. Mechanisms of mRNA degradation in eukaryotes. Trends Biochem Sci. 1994 Aug;19(8):336–340. doi: 10.1016/0968-0004(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Guldberg P., thor Straten P., Birck A., Ahrenkiel V., Kirkin A. F., Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res. 1997 Sep 1;57(17):3660–3663. [PubMed] [Google Scholar]
- Hanssen A. M., Fryns J. P. Cowden syndrome. J Med Genet. 1995 Feb;32(2):117–119. doi: 10.1136/jmg.32.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. Evidence for a relation of Epstein-Barr virus to Burkitt's lymphoma and nasopharyngeal carcinoma. Bibl Haematol. 1970;(36):706–713. doi: 10.1159/000391777. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Koi S., Fujimoto I., Hasumi K., Kato K., Nakamura Y. Allelotype of uterine cancer by analysis of RFLP and microsatellite polymorphisms: frequent loss of heterozygosity on chromosome arms 3p, 9q, 10q, and 17p. Genes Chromosomes Cancer. 1994 Feb;9(2):119–123. doi: 10.1002/gcc.2870090207. [DOI] [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991 Dec;5(12A):2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- Li D. M., Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997 Jun 1;57(11):2124–2129. [PubMed] [Google Scholar]
- Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997 Mar 28;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liaw D., Marsh D. J., Li J., Dahia P. L., Wang S. I., Zheng Z., Bose S., Call K. M., Tsou H. C., Peacocke M. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997 May;16(1):64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Lim S. K., Sigmund C. D., Gross K. W., Maquat L. E. Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products. Mol Cell Biol. 1992 Mar;12(3):1149–1161. doi: 10.1128/mcb.12.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E. Defects in RNA splicing and the consequence of shortened translational reading frames. Am J Hum Genet. 1996 Aug;59(2):279–286. [PMC free article] [PubMed] [Google Scholar]
- Marsh D. J., Dahia P. L., Zheng Z., Liaw D., Parsons R., Gorlin R. J., Eng C. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997 Aug;16(4):333–334. doi: 10.1038/ng0897-333. [DOI] [PubMed] [Google Scholar]
- Menon K. P., Neufeld E. F. Evidence for degradation of mRNA encoding alpha-L-iduronidase in Hurler fibroblasts with premature termination alleles. Cell Mol Biol (Noisy-le-grand) 1994 Nov;40(7):999–1005. [PubMed] [Google Scholar]
- Nelen M. R., van Staveren W. C., Peeters E. A., Hassel M. B., Gorlin R. J., Hamm H., Lindboe C. F., Fryns J. P., Sijmons R. H., Woods D. G. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997 Aug;6(8):1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- Pulak R., Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993 Oct;7(10):1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- Raskind W. H., Tirumali N., Jacobson R., Singer J., Fialkow P. J. Evidence for a multistep pathogenesis of a myelodysplastic syndrome. Blood. 1984 Jun;63(6):1318–1323. [PubMed] [Google Scholar]
- Rhei E., Kang L., Bogomolniy F., Federici M. G., Borgen P. I., Boyd J. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res. 1997 Sep 1;57(17):3657–3659. [PubMed] [Google Scholar]
- Steck P. A., Pershouse M. A., Jasser S. A., Yung W. K., Lin H., Ligon A. H., Langford L. A., Baumgard M. L., Hattier T., Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997 Apr;15(4):356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- Zedenius J., Wallin G., Svensson A., Bovèe J., Hög A., Bäckdahl M., Larsson C. Deletions of the long arm of chromosome 10 in progression of follicular thyroid tumors. Hum Genet. 1996 Mar;97(3):299–303. doi: 10.1007/BF02185758. [DOI] [PubMed] [Google Scholar]



