Abstract
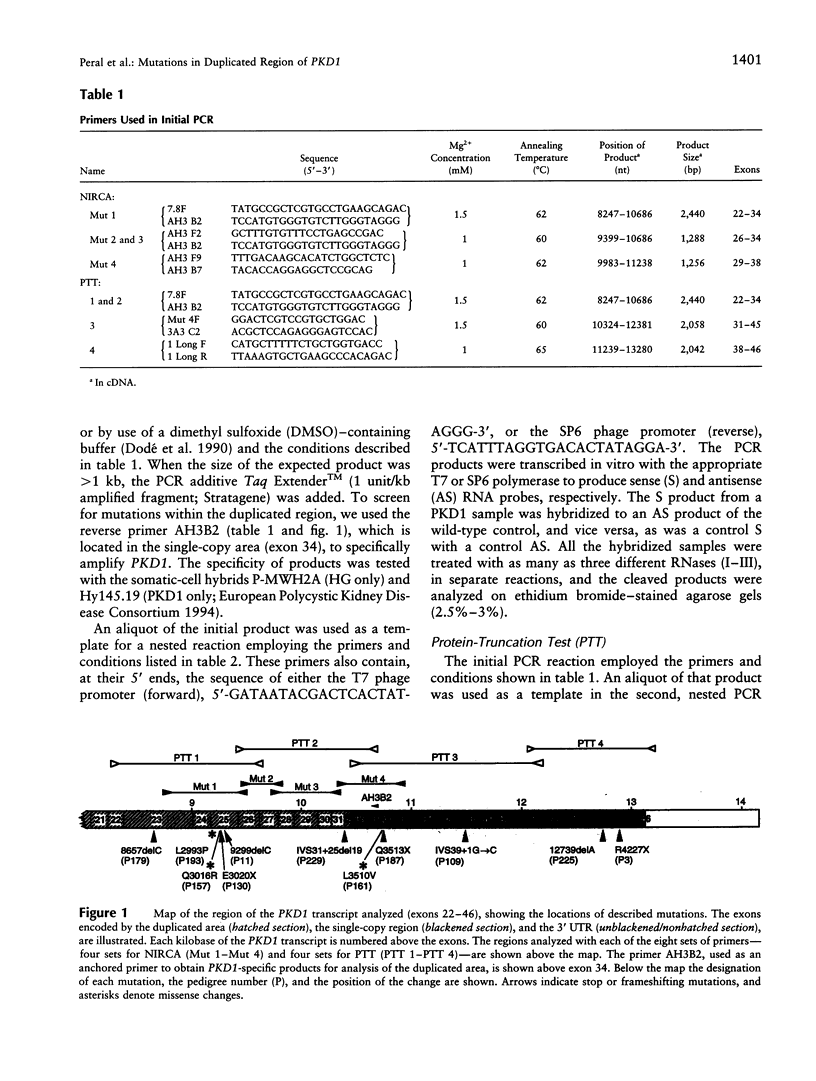
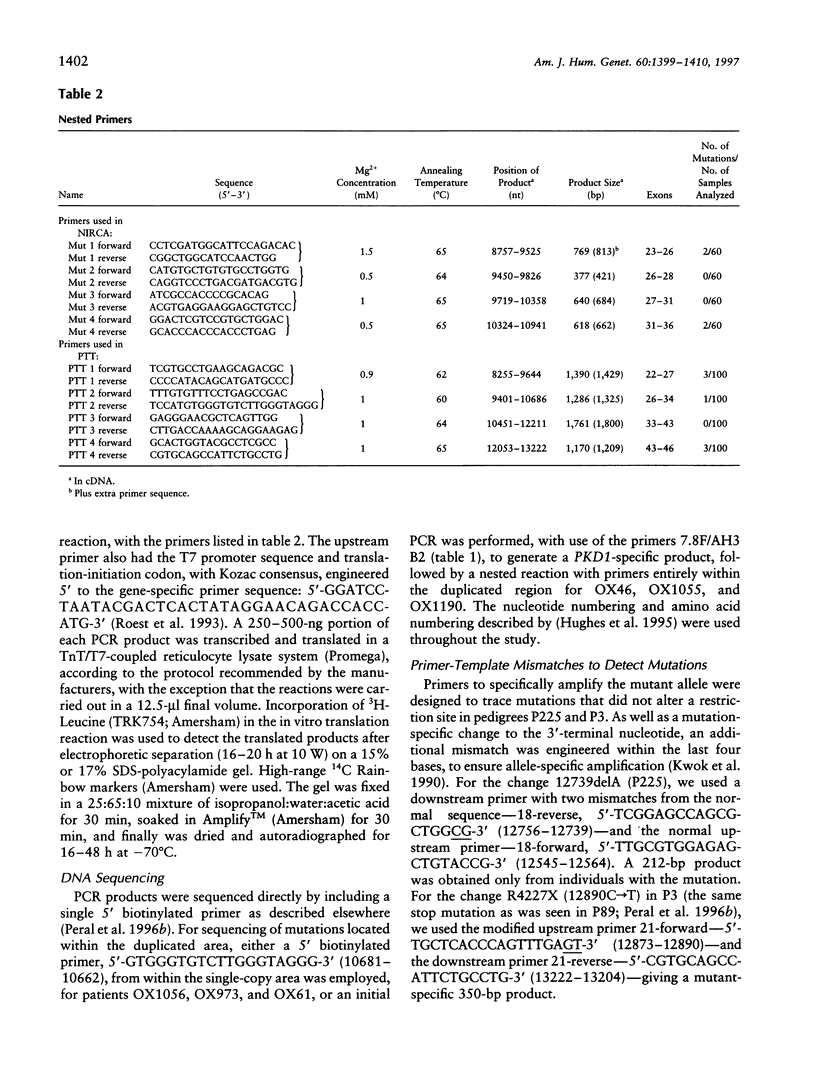
Mutation screening of the major autosomal dominant polycystic kidney disease gene (PKD1) has been complicated by the large transcript size (> 14 kb) and by reiteration of the genomic area encoding 75% of the protein on the same chromosome (the HG loci). The sequence similarity between the PKD1 and HG regions has precluded specific analysis of the duplicated region of PKD1, and consequently all previously described mutations map to the unique 3' region of PKD1. We have now developed a novel anchored reverse-transcription-PCR (RT-PCR) approach to specifically amplify duplicated regions of PKD1, employing one primer situated within the single-copy region and one within the reiterated area. This strategy has been incorporated in a mutation screen of 100 patients for more than half of the PKD1 exons (exons 22-46; 37% of the coding region), including 11 (exons 22-32) within the duplicated gene region, by use of the protein-truncation test (PTT). Sixty of these patients also were screened for missense changes, by use of the nonisotopic RNase cleavage assay (NIRCA), in exons 23-36. Eleven mutations have been identified, six within the duplicated region, and these consist of three stop mutations, three frameshifting deletions of a single nucleotide, two splicing defects, and three possible missense changes. Each mutation was detected in just one family (although one has been described elsewhere); no mutation hot spot was identified. The nature and distribution of mutations, plus the lack of a clear phenotype/genotype correlation, suggest that they may inactivate the molecule. RT-PCR/PTT proved to be a rapid and efficient method to detect PKD1 mutations (differentiating pathogenic changes from polymorphisms), and we recommend this procedure as a firstpass mutation screen in this disorder.
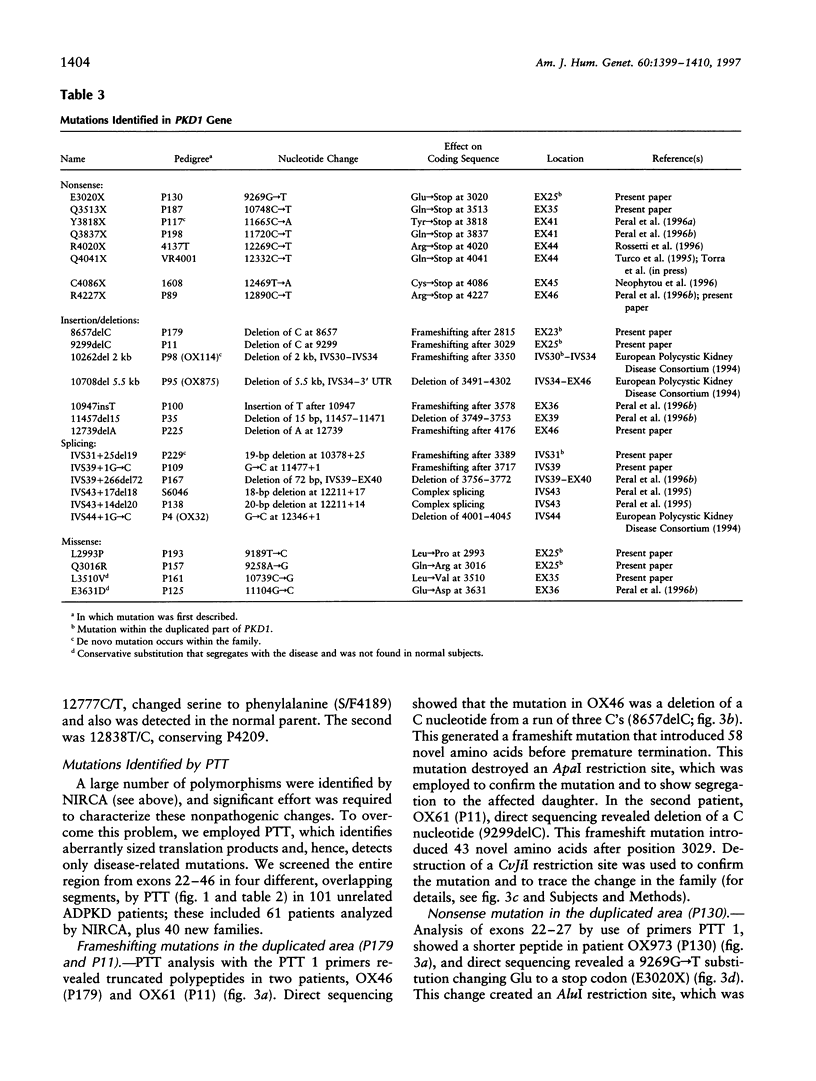
Full text
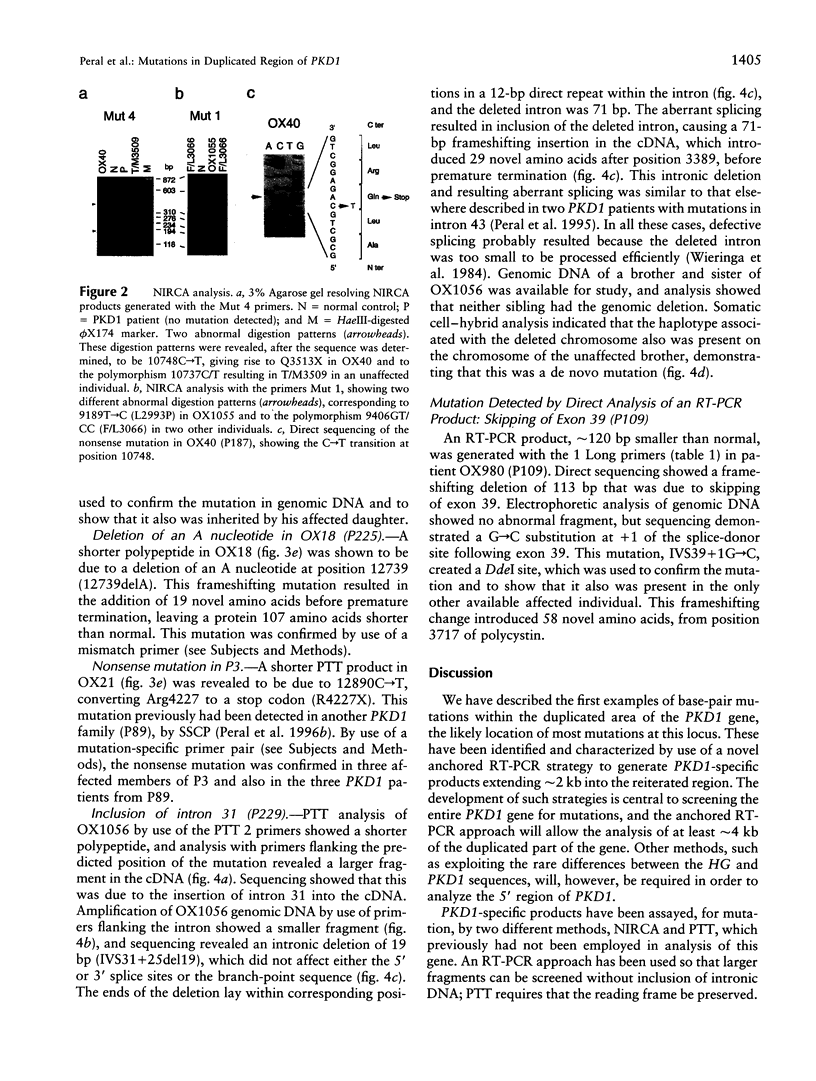
PDF


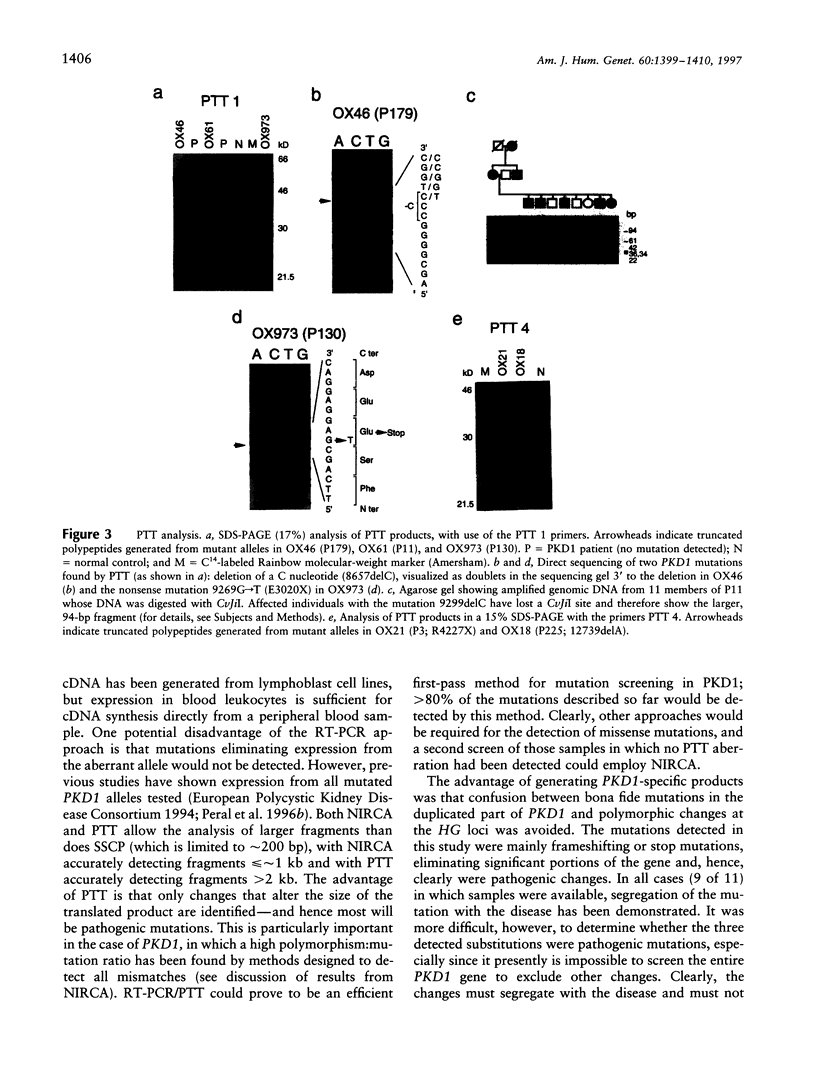



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksentijevich I., Pras E., Gruberg L., Shen Y., Holman K., Helling S., Prosen L., Sutherland G. R., Richards R. I., Ramsburg M. Refined mapping of the gene causing familial Mediterranean fever, by linkage and homozygosity studies. Am J Hum Genet. 1993 Aug;53(2):451–461. [PMC free article] [PubMed] [Google Scholar]
- Bogdanova N., Dworniczak B., Dragova D., Todorov V., Dimitrakov D., Kalinov K., Hallmayer J., Horst J., Kalaydjieva L. Genetic heterogeneity of polycystic kidney disease in Bulgaria. Hum Genet. 1995 Jun;95(6):645–650. doi: 10.1007/BF00209481. [DOI] [PubMed] [Google Scholar]
- Brasier J. L., Henske E. P. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J Clin Invest. 1997 Jan 15;99(2):194–199. doi: 10.1172/JCI119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook-Carter P. T., Peral B., Ward C. J., Thompson P., Hughes J., Maheshwar M. M., Nellist M., Gamble V., Harris P. C., Sampson J. R. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease--a contiguous gene syndrome. Nat Genet. 1994 Dec;8(4):328–332. doi: 10.1038/ng1294-328. [DOI] [PubMed] [Google Scholar]
- Burn T. C., Connors T. D., Dackowski W. R., Petry L. R., Van Raay T. J., Millholland J. M., Venet M., Miller G., Hakim R. M., Landes G. M. Analysis of the genomic sequence for the autosomal dominant polycystic kidney disease (PKD1) gene predicts the presence of a leucine-rich repeat. The American PKD1 Consortium (APKD1 Consortium). Hum Mol Genet. 1995 Apr;4(4):575–582. doi: 10.1093/hmg/4.4.575. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daoust M. C., Reynolds D. M., Bichet D. G., Somlo S. Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics. 1995 Feb 10;25(3):733–736. doi: 10.1016/0888-7543(95)80020-m. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Hendrick D. Activation of phenotypic expression of human globin genes from nonerythroid cells by chromosome-dependent transfer to tetraploid mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 May;76(5):2185–2189. doi: 10.1073/pnas.76.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodé C., Rochette J., Krishnamoorthy R. Locus assignment of human alpha globin mutations by selective amplification and direct sequencing. Br J Haematol. 1990 Oct;76(2):275–281. doi: 10.1111/j.1365-2141.1990.tb07884.x. [DOI] [PubMed] [Google Scholar]
- Gabow P. A. Autosomal dominant polycystic kidney disease--more than a renal disease. Am J Kidney Dis. 1990 Nov;16(5):403–413. doi: 10.1016/s0272-6386(12)80051-5. [DOI] [PubMed] [Google Scholar]
- Gabow P. A., Johnson A. M., Kaehny W. D., Kimberling W. J., Lezotte D. C., Duley I. T., Jones R. H. Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int. 1992 May;41(5):1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- Harris P. C., Thomas S., Ratcliffe P. J., Breuning M. H., Coto E., Lopez-Larrea C. Rapid genetic analysis of families with polycystic kidney disease 1 by means of a microsatellite marker. Lancet. 1991 Dec 14;338(8781):1484–1487. doi: 10.1016/0140-6736(91)92300-q. [DOI] [PubMed] [Google Scholar]
- Harris P. C., Ward C. J., Peral B., Hughes J. Polycystic kidney disease. 1: Identification and analysis of the primary defect. J Am Soc Nephrol. 1995 Oct;6(4):1125–1133. doi: 10.1681/ASN.V641125. [DOI] [PubMed] [Google Scholar]
- Hughes J., Ward C. J., Peral B., Aspinwall R., Clark K., San Millán J. L., Gamble V., Harris P. C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995 Jun;10(2):151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T., Wu G., Hayashi T., Xenophontos S. L., Veldhuisen B., Saris J. J., Reynolds D. M., Cai Y., Gabow P. A., Pierides A. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996 May 31;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- Moy G. W., Mendoza L. M., Schulz J. R., Swanson W. J., Glabe C. G., Vacquier V. D. The sea urchin sperm receptor for egg jelly is a modular protein with extensive homology to the human polycystic kidney disease protein, PKD1. J Cell Biol. 1996 May;133(4):809–817. doi: 10.1083/jcb.133.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Neophytou P., Constantinides R., Lazarou A., Pierides A., Deltas C. C. Detection of a novel nonsense mutation and an intragenic polymorphism in the PKD1 gene of a Cypriot family with autosomal dominant polycystic kidney disease. Hum Genet. 1996 Oct;98(4):437–442. doi: 10.1007/s004390050235. [DOI] [PubMed] [Google Scholar]
- Peral B., Gamble V., San Millán J. L., Strong C., Sloane-Stanley J., Moreno F., Harris P. C. Splicing mutations of the polycystic kidney disease 1 (PKD1) gene induced by intronic deletion. Hum Mol Genet. 1995 Apr;4(4):569–574. doi: 10.1093/hmg/4.4.569. [DOI] [PubMed] [Google Scholar]
- Peral B., Ong A. C., San Millán J. L., Gamble V., Rees L., Harris P. C. A stable, nonsense mutation associated with a case of infantile onset polycystic kidney disease 1 (PKD1). Hum Mol Genet. 1996 Apr;5(4):539–542. doi: 10.1093/hmg/5.4.539. [DOI] [PubMed] [Google Scholar]
- Peral B., San Millán J. L., Ong A. C., Gamble V., Ward C. J., Strong C., Harris P. C. Screening the 3' region of the polycystic kidney disease 1 (PKD1) gene reveals six novel mutations. Am J Hum Genet. 1996 Jan;58(1):86–96. [PMC free article] [PubMed] [Google Scholar]
- Peral B., Ward C. J., San Millán J. L., Thomas S., Stallings R. L., Moreno F., Harris P. C. Evidence of linkage disequilibrium in the Spanish polycystic kidney disease I population. Am J Hum Genet. 1994 May;54(5):899–908. [PMC free article] [PubMed] [Google Scholar]
- Peters D. J., Sandkuijl L. A. Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol. 1992;97:128–139. doi: 10.1159/000421651. [DOI] [PubMed] [Google Scholar]
- Ravine D., Walker R. G., Gibson R. N., Forrest S. M., Richards R. I., Friend K., Sheffield L. J., Kincaid-Smith P., Danks D. M. Phenotype and genotype heterogeneity in autosomal dominant polycystic kidney disease. Lancet. 1992 Nov 28;340(8831):1330–1333. doi: 10.1016/0140-6736(92)92503-8. [DOI] [PubMed] [Google Scholar]
- Roest P. A., Roberts R. G., Sugino S., van Ommen G. J., den Dunnen J. T. Protein truncation test (PTT) for rapid detection of translation-terminating mutations. Hum Mol Genet. 1993 Oct;2(10):1719–1721. doi: 10.1093/hmg/2.10.1719. [DOI] [PubMed] [Google Scholar]
- Rossetti S., Bresin E., Restagno G., Carbonara A., Corrà S., De Prisco O., Pignatti P. F., Turco A. E. Autosomal dominant polycystic kidney disease (ADPKD) in an Italian family carrying a novel nonsense mutation and two missense changes in exons 44 and 45 of the PKD1 Gene. Am J Med Genet. 1996 Oct 16;65(2):155–159. doi: 10.1002/(SICI)1096-8628(19961016)65:2<155::AID-AJMG15>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Snarey A., Thomas S., Schneider M. C., Pound S. E., Barton N., Wright A. F., Somlo S., Germino G. G., Harris P. C., Reeders S. T. Linkage disequilibrium in the region of the autosomal dominant polycystic kidney disease gene (PKD1). Am J Hum Genet. 1994 Aug;55(2):365–371. [PMC free article] [PubMed] [Google Scholar]
- Turco A. E., Rossetti S., Bresin E., Corra S., Gammaro L., Maschio G., Pignatti P. F. A novel nonsense mutation in the PKD1 gene (C3817T) is associated with autosomal dominant polycystic kidney disease (ADPKD) in a large three-generation Italian family. Hum Mol Genet. 1995 Aug;4(8):1331–1335. doi: 10.1093/hmg/4.8.1331. [DOI] [PubMed] [Google Scholar]
- White P. C., Tusie-Luna M. T., New M. I., Speiser P. W. Mutations in steroid 21-hydroxylase (CYP21). Hum Mutat. 1994;3(4):373–378. doi: 10.1002/humu.1380030408. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Winter E., Yamamoto F., Almoguera C., Perucho M. A method to detect and characterize point mutations in transcribed genes: amplification and overexpression of the mutant c-Ki-ras allele in human tumor cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7575–7579. doi: 10.1073/pnas.82.22.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida S., de Almeida E., Peters D., Pinto J. R., Távora I., Lavinha J., Breuning M., Prata M. M. Autosomal dominant polycystic kidney disease: evidence for the existence of a third locus in a Portuguese family. Hum Genet. 1995 Jul;96(1):83–88. doi: 10.1007/BF00214191. [DOI] [PubMed] [Google Scholar]