Abstract
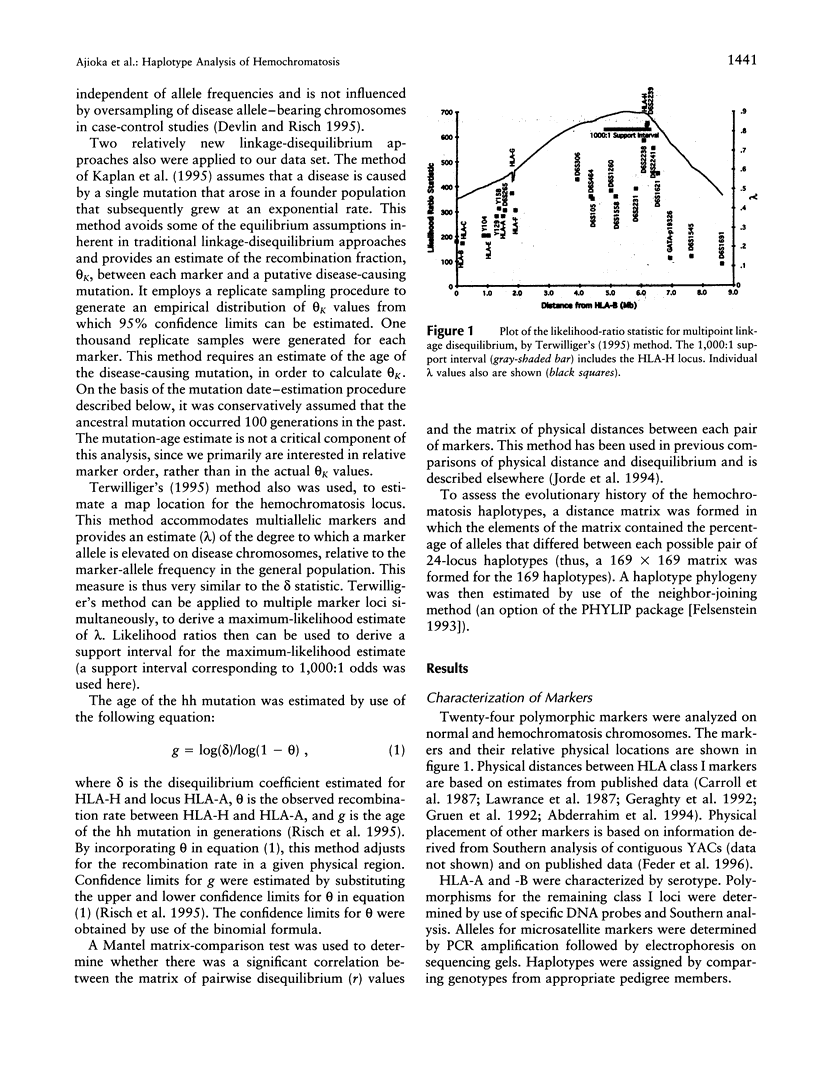
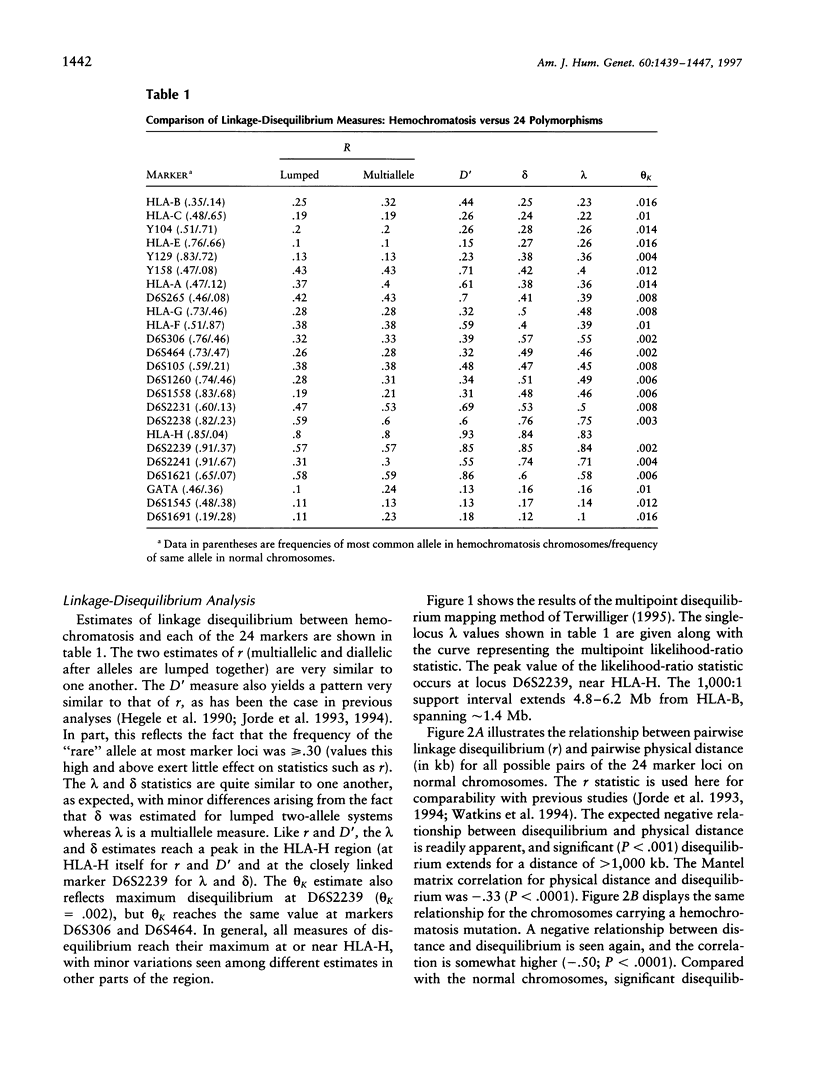
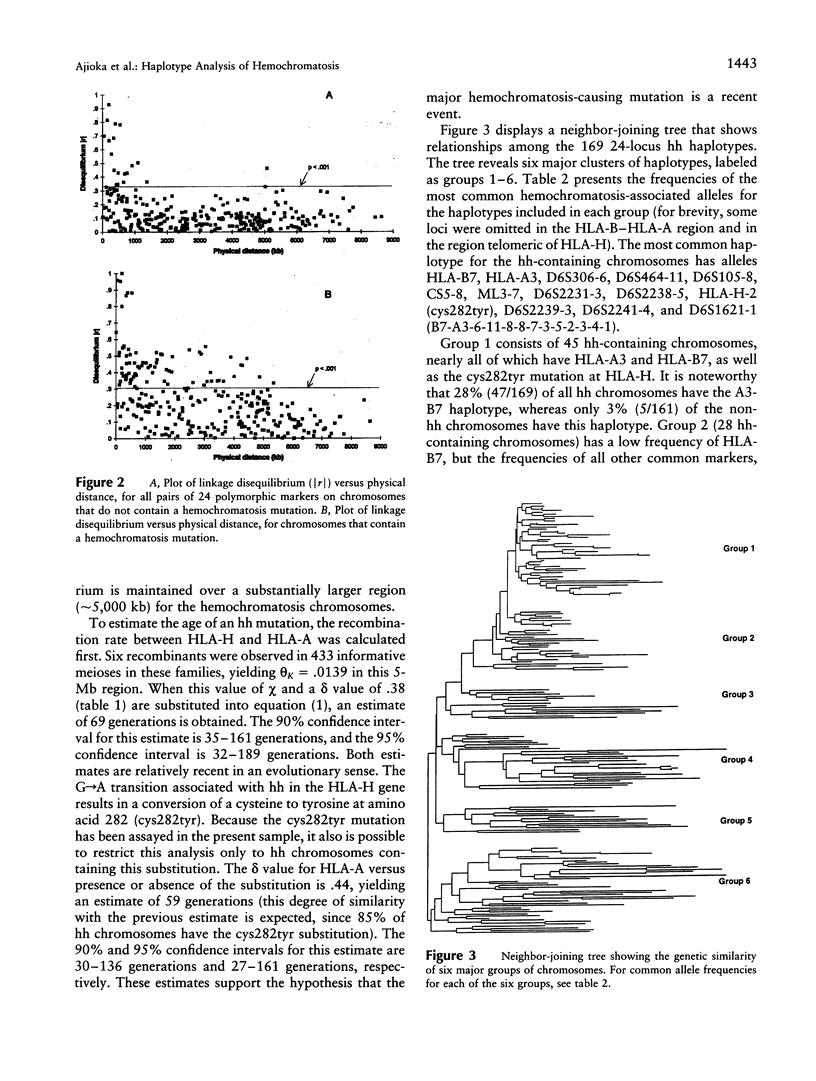
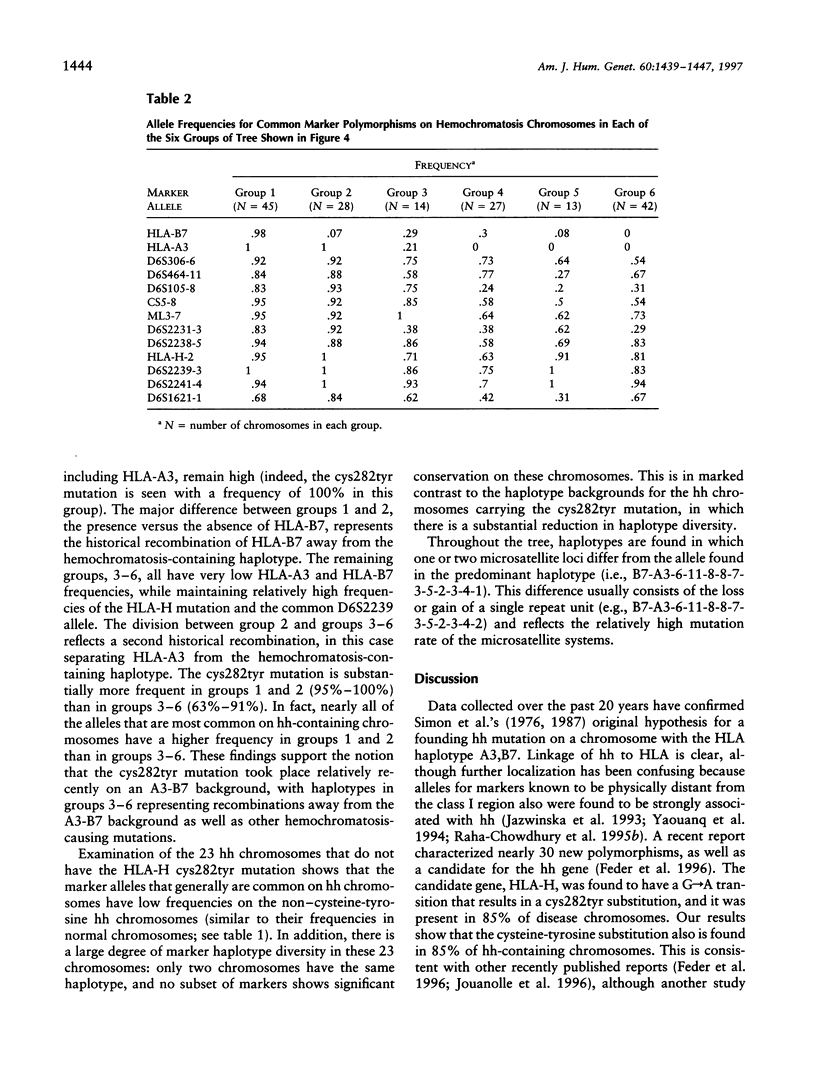
We applied several types of linkage-disequilibrium calculations to analyze the hereditary hemochromatosis (hh) locus. Twenty-four polymorphic markers in the major histocompatibility complex (MHC) class I region were used to haplotype hh and normal chromosomes. A total of 169 hh and 161 normal chromosomes were analyzed. Disequilibrium values were found to be high over an unusually large region beginning 150 kb centromeric of HLA-A and extending nearly 5 Mb telomeric of it. Recombination in this region was approximately 28% of the expected value. This low level of recombination contributes to the unusually broad region of linkage disequilibrium found with hh. The strongest disequilibrium was found at locus HLA-H (delta = .84) and at locus D6S2239 (delta = .85), a marker approximately 10 kb telomeric to HLA-H. All disequilibrium methods employed in this study found peak disequilibrium at HLA-H or D6S2239. The cys282tyr mutation in HLA-H, a candidate gene for hh, was found in 85% of disease chromosomes. A haplotype phylogeny for hh chromosomes was constructed and suggests that the mutation associated with the most common haplotype occurred relatively recently. The age of the hh mutation was estimated to be approximately 60-70 generations. Disequilibrium was maintained over a greater distance for hh-carrying chromosomes, consistent with a recent mutation for hh. Our data provide a reasonable explanation for previous difficulties in localizing the hh locus and provide an evolutionary history for disease chromosomes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abderrahim H., Sambucy J. L., Iris F., Ougen P., Billault A., Chumakov I. M., Dausset J., Cohen D., Le Paslier D. Cloning the human major histocompatibility complex in YACs. Genomics. 1994 Oct;23(3):520–527. doi: 10.1006/geno.1994.1538. [DOI] [PubMed] [Google Scholar]
- Bengtsson B. O., Thomson G. Measuring the strength of associations between HLA antigens and diseases. Tissue Antigens. 1981 Nov;18(5):356–363. doi: 10.1111/j.1399-0039.1981.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Katzman P., Alicot E. M., Koller B. H., Geraghty D. E., Orr H. T., Strominger J. L., Spies T. Linkage map of the human major histocompatibility complex including the tumor necrosis factor genes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8535–8539. doi: 10.1073/pnas.84.23.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright G. E., Skolnick M., Amos D. B., Edwards C. Q., Kravitz K., Johnson A. Inheritance of hemochromatosis: linkage to HLA. Trans Assoc Am Physicians. 1978;91:273–281. [PubMed] [Google Scholar]
- Crawford D. H., Powell L. W., Leggett B. A., Francis J. S., Fletcher L. M., Webb S. I., Halliday J. W., Jazwinska E. C. Evidence that the ancestral haplotype in Australian hemochromatosis patients may be associated with a common mutation in the gene. Am J Hum Genet. 1995 Aug;57(2):362–367. [PMC free article] [PubMed] [Google Scholar]
- Cutting G. R., Kasch L. M., Rosenstein B. J., Zielenski J., Tsui L. C., Antonarakis S. E., Kazazian H. H., Jr A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990 Jul 26;346(6282):366–369. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- Dadone M. M., Kushner J. P., Edwards C. Q., Bishop D. T., Skolnick M. H. Hereditary hemochromatosis. Analysis of laboratory expression of the disease by genotype in 18 pedigrees. Am J Clin Pathol. 1982 Aug;78(2):196–207. doi: 10.1093/ajcp/78.2.196. [DOI] [PubMed] [Google Scholar]
- Devlin B., Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995 Sep 20;29(2):311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- Edwards C. Q., Dadone M. M., Skolnick M. H., Kushner J. P. Hereditary haemochromatosis. Clin Haematol. 1982 Jun;11(2):411–435. [PubMed] [Google Scholar]
- Edwards C. Q., Griffen L. M., Goldgar D., Drummond C., Skolnick M. H., Kushner J. P. Prevalence of hemochromatosis among 11,065 presumably healthy blood donors. N Engl J Med. 1988 May 26;318(21):1355–1362. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- Edwards C. Q., Skolnick M. H., Kushner J. P. Hereditary hemochromatosis: contributions of genetic analyses. Prog Hematol. 1981;12:43–71. [PubMed] [Google Scholar]
- Eisensmith R. C., Okano Y., Dasovich M., Wang T., Güttler F., Lou H., Guldberg P., Lichter-Konecki U., Konecki D. S., Svensson E. Multiple origins for phenylketonuria in Europe. Am J Hum Genet. 1992 Dec;51(6):1355–1365. [PMC free article] [PubMed] [Google Scholar]
- Feder J. N., Gnirke A., Thomas W., Tsuchihashi Z., Ruddy D. A., Basava A., Dormishian F., Domingo R., Jr, Ellis M. C., Fullan A. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996 Aug;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Orr H. T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty D. E., Pei J., Lipsky B., Hansen J. A., Taillon-Miller P., Bronson S. K., Chaplin D. D. Cloning and physical mapping of the HLA class I region spanning the HLA-E-to-HLA-F interval by using yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2669–2673. doi: 10.1073/pnas.89.7.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen J. R., Goei V. L., Summers K. M., Capossela A., Powell L., Halliday J., Zoghbi H., Shukla H., Weissman S. M. Physical and genetic mapping of the telomeric major histocompatibility complex region in man and relevance to the primary hemochromatosis gene (HFE). Genomics. 1992 Oct;14(2):232–240. doi: 10.1016/s0888-7543(05)80211-3. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Floyd J., Rundle S. A., Crow S., Walsh K. V., Thibault M. C., Harper P. S., Shaw D. J. Detection of linkage disequilibrium between the myotonic dystrophy locus and a new polymorphic DNA marker. Am J Hum Genet. 1991 Jul;49(1):68–75. [PMC free article] [PubMed] [Google Scholar]
- Hedrick P. W. Gametic disequilibrium measures: proceed with caution. Genetics. 1987 Oct;117(2):331–341. doi: 10.1093/genetics/117.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele R. A., Plaetke R., Lalouel J. M. Linkage disequilibrium between DNA markers at the low-density lipoprotein receptor gene. Genet Epidemiol. 1990;7(1):69–81. doi: 10.1002/gepi.1370070114. [DOI] [PubMed] [Google Scholar]
- Jazwinska E. C., Cullen L. M., Busfield F., Pyper W. R., Webb S. I., Powell L. W., Morris C. P., Walsh T. P. Haemochromatosis and HLA-H. Nat Genet. 1996 Nov;14(3):249–251. doi: 10.1038/ng1196-249. [DOI] [PubMed] [Google Scholar]
- Jazwinska E. C., Lee S. C., Webb S. I., Halliday J. W., Powell L. W. Localization of the hemochromatosis gene close to D6S105. Am J Hum Genet. 1993 Aug;53(2):347–352. [PMC free article] [PubMed] [Google Scholar]
- Jazwinska E. C., Pyper W. R., Burt M. J., Francis J. L., Goldwurm S., Webb S. I., Lee S. C., Halliday J. W., Powell L. W. Haplotype analysis in Australian hemochromatosis patients: evidence for a predominant ancestral haplotype exclusively associated with hemochromatosis. Am J Hum Genet. 1995 Feb;56(2):428–433. [PMC free article] [PubMed] [Google Scholar]
- Jorde L. B. Linkage disequilibrium as a gene-mapping tool. Am J Hum Genet. 1995 Jan;56(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Jorde L. B., Watkins W. S., Carlson M., Groden J., Albertsen H., Thliveris A., Leppert M. Linkage disequilibrium predicts physical distance in the adenomatous polyposis coli region. Am J Hum Genet. 1994 May;54(5):884–898. [PMC free article] [PubMed] [Google Scholar]
- Jorde L. B., Watkins W. S., Viskochil D., O'Connell P., Ward K. Linkage disequilibrium in the neurofibromatosis 1 (NF1) region: implications for gene mapping. Am J Hum Genet. 1993 Nov;53(5):1038–1050. [PMC free article] [PubMed] [Google Scholar]
- Jouanolle A. M., Gandon G., Jézéquel P., Blayau M., Campion M. L., Yaouanq J., Mosser J., Fergelot P., Chauvel B., Bouric P. Haemochromatosis and HLA-H. Nat Genet. 1996 Nov;14(3):251–252. doi: 10.1038/ng1196-251. [DOI] [PubMed] [Google Scholar]
- Kaplan N. L., Hill W. G., Weir B. S. Likelihood methods for locating disease genes in nonequilibrium populations. Am J Hum Genet. 1995 Jan;56(1):18–32. [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Geraghty D. E., DeMars R., Duvick L., Rich S. S., Orr H. T. Chromosomal organization of the human major histocompatibility complex class I gene family. J Exp Med. 1989 Feb 1;169(2):469–480. doi: 10.1084/jem.169.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance S. K., Smith C. L., Srivastava R., Cantor C. R., Weissman S. M. Megabase-scale mapping of the HLA gene complex by pulsed field gel electrophoresis. Science. 1987 Mar 13;235(4794):1387–1390. doi: 10.1126/science.3029868. [DOI] [PubMed] [Google Scholar]
- Lewontin R C. The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics. 1964 Jan;49(1):49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Hors J., Saleun J. P., Saddi R., Passa P., Lafaurie S., Feingold N., Dausset J. Idiopathic hemochromatosis: linkage with HLA. Tissue Antigens. 1978 May;11(5):471–474. doi: 10.1111/j.1399-0039.1978.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Martin M., Mann D., Carrington M. Recombination rates across the HLA complex: use of microsatellites as a rapid screen for recombinant chromosomes. Hum Mol Genet. 1995 Mar;4(3):423–428. doi: 10.1093/hmg/4.3.423. [DOI] [PubMed] [Google Scholar]
- McLellan T., Jorde L. B., Skolnick M. H. Genetic distances between the Utah Mormons and related populations. Am J Hum Genet. 1984 Jul;36(4):836–857. [PMC free article] [PubMed] [Google Scholar]
- Petrukhin K., Fischer S. G., Pirastu M., Tanzi R. E., Chernov I., Devoto M., Brzustowicz L. M., Cayanis E., Vitale E., Russo J. J. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993 Dec;5(4):338–343. doi: 10.1038/ng1293-338. [DOI] [PubMed] [Google Scholar]
- Raha-Chowdhury R., Bowen D. J., Burnett A. K., Worwood M. Allelic associations and homozygosity at loci from HLA-B to D6S299 in genetic haemochromatosis. J Med Genet. 1995 Jun;32(6):446–452. doi: 10.1136/jmg.32.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha-Chowdhury R., Bowen D. J., Stone C., Pointon J. J., Terwilliger J. D., Shearman J. D., Robson K. J., Bomford A., Worwood M. New polymorphic microsatellite markers place the haemochromatosis gene telomeric to D6S105. Hum Mol Genet. 1995 Oct;4(10):1869–1874. doi: 10.1093/hmg/4.10.1869. [DOI] [PubMed] [Google Scholar]
- Risch N., de Leon D., Ozelius L., Kramer P., Almasy L., Singer B., Fahn S., Breakefield X., Bressman S. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet. 1995 Feb;9(2):152–159. doi: 10.1038/ng0295-152. [DOI] [PubMed] [Google Scholar]
- Simon M., Bourel M., Fauchet R., Genetet B. Association of HLA-A3 and HLA-B14 antigens with idiopathic haemochromatosis. Gut. 1976 May;17(5):332–334. doi: 10.1136/gut.17.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M., Bourel M., Genetet B., Fauchet R. Idiopathic hemochromatosis. Demonstration of recessive transmission and early detection by family HLA typing. N Engl J Med. 1977 Nov 10;297(19):1017–1021. doi: 10.1056/NEJM197711102971901. [DOI] [PubMed] [Google Scholar]
- Simon M., Le Mignon L., Fauchet R., Yaouanq J., David V., Edan G., Bourel M. A study of 609 HLA haplotypes marking for the hemochromatosis gene: (1) mapping of the gene near the HLA-A locus and characters required to define a heterozygous population and (2) hypothesis concerning the underlying cause of hemochromatosis-HLA association. Am J Hum Genet. 1987 Aug;41(2):89–105. [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Haigh J. The hitch-hiking effect of a favourable gene. Genet Res. 1974 Feb;23(1):23–35. [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger J. D. A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. Am J Hum Genet. 1995 Mar;56(3):777–787. [PMC free article] [PubMed] [Google Scholar]
- Watkins W. S., Zenger R., O'Brien E., Nyman D., Eriksson A. W., Renlund M., Jorde L. B. Linkage disequilibrium patterns vary with chromosomal location: a case study from the von Willebrand factor region. Am J Hum Genet. 1994 Aug;55(2):348–355. [PMC free article] [PubMed] [Google Scholar]
- Worwood M., Raha-Chowdhury R., Dorak M. T., Darke C., Bowen D. J., Burnett A. K. Alleles at D6S265 and D6S105 define a haemochromatosis-specific genotype. Br J Haematol. 1994 Apr;86(4):863–866. doi: 10.1111/j.1365-2141.1994.tb04843.x. [DOI] [PubMed] [Google Scholar]
- Yaouanq J., Perichon M., Chorney M., Pontarotti P., Le Treut A., el Kahloun A., Mauvieux V., Blayau M., Jouanolle A. M., Chauvel B. Anonymous marker loci within 400 kb of HLA-A generate haplotypes in linkage disequilibrium with the hemochromatosis gene (HFE) Am J Hum Genet. 1994 Feb;54(2):252–263. [PMC free article] [PubMed] [Google Scholar]


