Abstract
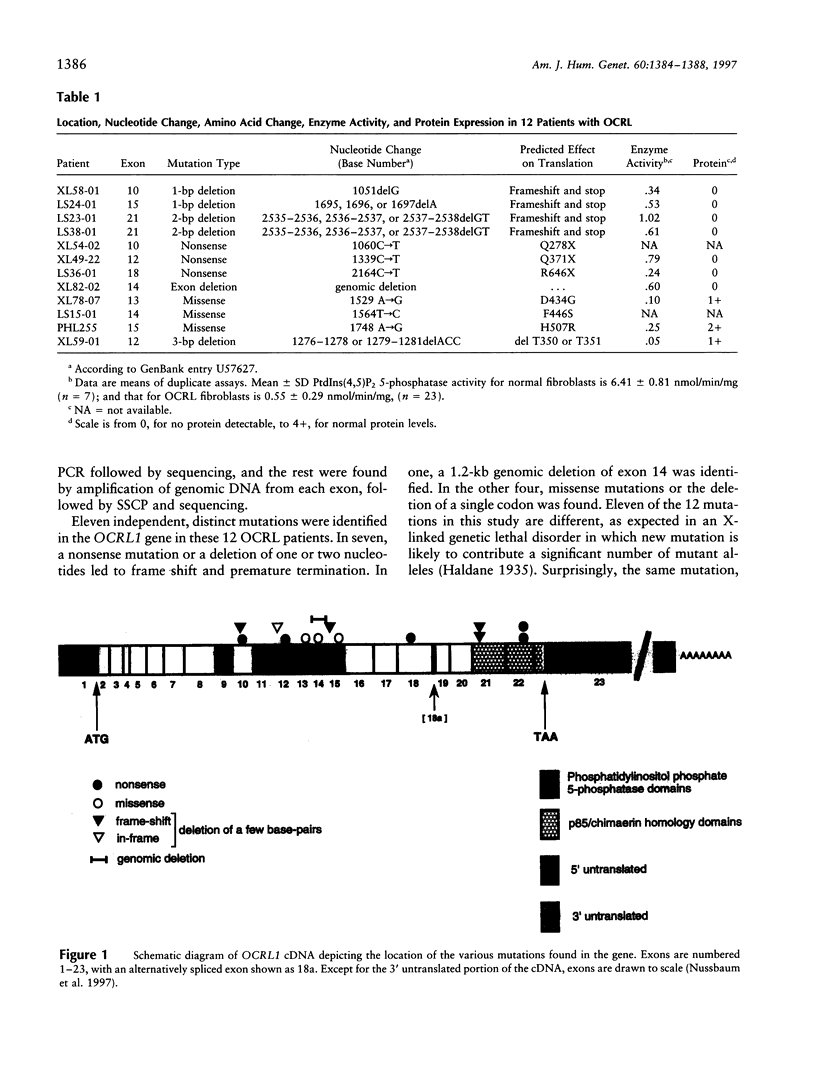
The oculocerebrorenal syndrome of Lowe (OCRL) is a multisystem disorder characterized by congenital cataracts, mental retardation, and renal Fanconi syndrome. The OCRL1 gene, which, when mutated, is responsible for OCRL, encodes a 105-kD Golgi protein with phosphatidylinositol (4,5)bisphosphate (PtdIn[4,5]P2) 5-phosphatase activity. We have examined the OCRL1 gene in 12 independent patients with OCRL and have found 11 different mutations. Six were nonsense mutations, and one a deletion of one or two nucleotides that leads to frameshift and premature termination. In one, a 1.2-kb genomic deletion of exon 14 was identified. In four others, missense mutations or the deletion of a single codon were found to involve amino acid residues known to be highly conserved among proteins with PtdIns(4,5)P2 5-phosphatase activity. All patients had markedly reduced PtdIns(4,5)P2 5-phosphatase activity in their fibroblasts, whereas the ocrl1 protein was detectable by immunoblotting in some patients with either missense mutations or a codon deletion but was not detectable in those with premature termination mutations. These results confirm and extend our previous observation that the OCRL phenotype results from loss of function of the ocrl1 protein and that mutations are generally heterogeneous. Missense mutations that abolish enzyme activity but not expression of the protein will be useful for studying structure-function relationships in PtdIns(4,5)P2 5-phosphatases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attree O., Olivos I. M., Okabe I., Bailey L. C., Nelson D. L., Lewis R. A., McInnes R. R., Nussbaum R. L. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992 Jul 16;358(6383):239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Performance evaluation of amino acid substitution matrices. Proteins. 1993 Sep;17(1):49–61. doi: 10.1002/prot.340170108. [DOI] [PubMed] [Google Scholar]
- Hodgson S. V., Heckmatt J. Z., Hughes E., Crolla J. A., Dubowitz V., Bobrow M. A balanced de novo X/autosome translocation in a girl with manifestations of Lowe syndrome. Am J Med Genet. 1986 Mar;23(3):837–847. doi: 10.1002/ajmg.1320230311. [DOI] [PubMed] [Google Scholar]
- Jefferson A. B., Majerus P. W. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem. 1995 Apr 21;270(16):9370–9377. doi: 10.1074/jbc.270.16.9370. [DOI] [PubMed] [Google Scholar]
- LOWE C. U., TERREY M., MacLACHLAN E. A. Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child. 1952 Feb;83(2):164–184. doi: 10.1001/archpedi.1952.02040060030004. [DOI] [PubMed] [Google Scholar]
- Leahey A. M., Charnas L. R., Nussbaum R. L. Nonsense mutations in the OCRL-1 gene in patients with the oculocerebrorenal syndrome of Lowe. Hum Mol Genet. 1993 Apr;2(4):461–463. doi: 10.1093/hmg/2.4.461. [DOI] [PubMed] [Google Scholar]
- Matzaris M., Jackson S. P., Laxminarayan K. M., Speed C. J., Mitchell C. A. Identification and characterization of the phosphatidylinositol-(4, 5)-bisphosphate 5-phosphatase in human platelets. J Biol Chem. 1994 Feb 4;269(5):3397–3402. [PubMed] [Google Scholar]
- McPherson P. S., Garcia E. P., Slepnev V. I., David C., Zhang X., Grabs D., Sossin W. S., Bauerfeind R., Nemoto Y., De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996 Jan 25;379(6563):353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Mueller O. T., Hartsfield J. K., Jr, Gallardo L. A., Essig Y. P., Miller K. L., Papenhausen P. R., Tedesco T. A. Lowe oculocerebrorenal syndrome in a female with a balanced X;20 translocation: mapping of the X chromosome breakpoint. Am J Hum Genet. 1991 Oct;49(4):804–810. [PMC free article] [PubMed] [Google Scholar]
- Nussbaum R. L., Orrison B. M., Jänne P. A., Charnas L., Chinault A. C. Physical mapping and genomic structure of the Lowe syndrome gene OCRL1. Hum Genet. 1997 Feb;99(2):145–150. doi: 10.1007/s004390050329. [DOI] [PubMed] [Google Scholar]
- Olivos-Glander I. M., Jänne P. A., Nussbaum R. L. The oculocerebrorenal syndrome gene product is a 105-kD protein localized to the Golgi complex. Am J Hum Genet. 1995 Oct;57(4):817–823. [PMC free article] [PubMed] [Google Scholar]
- Ross T. S., Jefferson A. B., Mitchell C. A., Majerus P. W. Cloning and expression of human 75-kDa inositol polyphosphate-5-phosphatase. J Biol Chem. 1991 Oct 25;266(30):20283–20289. [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Suchy S. F., Olivos-Glander I. M., Nussabaum R. L. Lowe syndrome, a deficiency of phosphatidylinositol 4,5-bisphosphate 5-phosphatase in the Golgi apparatus. Hum Mol Genet. 1995 Dec;4(12):2245–2250. doi: 10.1093/hmg/4.12.2245. [DOI] [PubMed] [Google Scholar]
- Zhang X., Jefferson A. B., Auethavekiat V., Majerus P. W. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]


