Abstract
Glucokinase (Gck) functions as a glucose sensor for insulin secretion, and in mice fed standard chow, haploinsufficiency of β cell–specific Gck (Gck+/–) causes impaired insulin secretion to glucose, although the animals have a normal β cell mass. When fed a high-fat (HF) diet, wild-type mice showed marked β cell hyperplasia, whereas Gck+/– mice demonstrated decreased β cell replication and insufficient β cell hyperplasia despite showing a similar degree of insulin resistance. DNA chip analysis revealed decreased insulin receptor substrate 2 (Irs2) expression in HF diet–fed Gck+/– mouse islets compared with wild-type islets. Western blot analyses confirmed upregulated Irs2 expression in the islets of HF diet–fed wild-type mice compared with those fed standard chow and reduced expression in HF diet–fed Gck+/– mice compared with those of HF diet–fed wild-type mice. HF diet–fed Irs2+/– mice failed to show a sufficient increase in β cell mass, and overexpression of Irs2 in β cells of HF diet–fed Gck+/– mice partially prevented diabetes by increasing β cell mass. These results suggest that Gck and Irs2 are critical requirements for β cell hyperplasia to occur in response to HF diet–induced insulin resistance.
Introduction
Glucokinase (Gck) catalyzes the conversion of glucose into glucose-6-phosphate, and more than 30 years ago Matschinsky and Ellerman proposed that it is critical for glucose sensing (1). Spontaneous inactivating mutations of the Gck gene coupled with autosomal-dominant inheritance patterns have been identified in maturity-onset diabetes of the young (MODY) patients (2). These mutations are found in the regions common to the β cell–specific isoform and liver isoform, and both decreased β cell function and impaired glucose uptake by the liver have been suggested to be involved in the pathogenesis of MODY (2). We have previously shown that haploinsufficiency of β cell–specific Gck (Gck+/–) leads to mild diabetes associated with impaired insulin secretion in response to glucose (3). Gck is now recognized as functioning as a glucose sensor for insulin secretion by pancreatic β cells (4).
Human type 2 diabetes is characterized by 2 major features: peripheral insulin resistance and impaired insulin secretion by pancreatic β cells (5, 6). Blood glucose levels are maintained within the normal range by adaptations of β cell mass and/or function as a compensatory response to insulin resistance. It should be noted that only 15%–20% of obese or severely insulin-resistant subjects become diabetic; the others maintain normoglycemia via β cell compensation (7). Diabetes only develops when insulin secretion by β cells is insufficient to compensate for the insulin resistance. For example, while both insulin receptor substrate 1 (IRS-1) knockout (Irs1–/–) mice (8, 9) and IRS-2 knockout (Irs2–/–) mice (10, 11) exhibit similar degrees of insulin resistance, Irs1–/– mice have normal glucose tolerance as a result of compensatory β cell hyperplasia, whereas Irs2–/– mice develop diabetes because of a lack of β cell hyperplasia in response to insulin resistance. The prevalence of diabetes has increased markedly in both Western countries and Japan, and the increase can be explained by drastic changes in lifestyle, such as a high-fat diet and sedentary lifestyle. Hypertrophic adipocytes produce an excess of hormones and nutrients that have been reported to cause insulin resistance, such as FFAs (12), and secrete less adiponectin, which has been reported to increase insulin sensitivity (13). Tumor necrosis factor α is secreted by macrophages residing within hypertrophied adipose tissue and causes insulin resistance (14). Under insulin-resistant conditions, blood glucose levels are maintained within the normal range by adaptations of β cell mass (hyperplasia) (8, 9). Glucose itself (7); insulin (15); Igfs (16); transcription factors such as insulin promoter factor 1 (Ipf1) and FoxO1 (refs. 17, 18); tyrosine kinase pathways including the insulin receptor (Insr) (19), Igf1 receptor (Igf1r) (19), Irs2 (10, 11), and Akt (20, 21) pathways; the prolactin signaling pathway (22); and Hgf (23) have been reported to be implicated in β cell growth; however, coordinated regulation of β cell mass by these factors under high-fat (HF) diet–induced insulin-resistant conditions has not been fully elucidated. To establish an animal model representative of human type 2 diabetes, we fed wild-type mice and Gck+/– mice a HF diet and investigated their glucose homeostasis and β cell mass and function. On the HF diet, wild-type mice showed marked β cell hyperplasia, whereas Gck+/– mice demonstrated insufficient β cell hyperplasia despite the presence of a similar degree of insulin resistance. Additionally, expression of Irs2 was upregulated in the islets of wild-type mice on the HF diet but markedly lower in those of HF diet–fed Gck+/– mice, and overexpression of Irs2 in β cells of HF diet–fed Gck+/– mice partially prevented diabetes by increasing β cell mass. Thus, Gck and Irs2 are critical requirements for β cell hyperplasia to occur in response to HF diet–induced insulin resistance.
Results
Early development of diabetes in Gck+/– mice on a HF diet.
When we fed 8-week-old male wild-type and Gck+/– mice a HF diet or standard chow, the 2 genotypes showed similar amounts of body weight gain, adipocyte size, and serum FFA levels that were significantly greater on the HF diet than on standard chow (Figure 1, A–D). After 20 weeks, the glucose-lowering effect of insulin was markedly impaired in both groups on the HF diet compared with those fed standard chow (Figure 1E). After 4 weeks on the HF diet, the wild-type mice maintained normal glucose tolerance as a result of compensatory hyperinsulinemia, whereas the Gck+/– mice developed severe diabetes because of a lack of compensatory hyperinsulinemia (Figure 1, F and G, left panels), despite the similar degrees of obesity and insulin resistance shown by both HF diet–fed groups. Although the wild-type mice on the HF diet developed mild diabetes by 20 weeks, because of the compensatory hyperinsulinemia they never developed severe diabetes (Figure 1, F and G, right panels). After 20 weeks, the insulin-to-glucose ratio 30 minutes after a glucose load (the insulin secretory index) revealed a significantly increased insulin response to glucose in the wild-type mice on the HF diet compared with those fed standard chow, whereas the Gck+/– mice on the HF diet had an insulin secretory index similar to that of the Gck+/– mice on standard chow (Figure 1H), indicating that no compensatory increase in insulin secretion occurred in the Gck+/– mice while on the HF diet.
Figure 1. Development of diabetes in Gck+/– mice fed HF diet.
(A and B) Body weight (A) and total weight of white adipose tissue (epididymal and retroperitoneal fat pads) (B) in wild-type and Gck+/– mice after 20 weeks on a standard chow or HF diet (n = 17–20). (C) Cell size in epididymal white adipose tissue (n = 600–900). (D) Serum FFA levels (n = 8–10). (E) Insulin tolerance in wild-type and Gck+/– mice after 4 weeks (left) and 20 weeks (right) on a standard chow or HF diet. Mice were given free access to food and then intraperitoneally injected with 0.75 mU of human insulin per gram body weight. n = 8 (standard chow–fed wild-type), 9 (HF diet–fed wild-type), 12 (standard chow–fed Gck+/–), 14 (HF diet–fed Gck+/–). (F and G) Glucose tolerance in wild-type and Gck+/– mice after 4 and 20 weeks on standard chow or HF diet. (F) Plasma glucose levels. (G) Serum insulin levels. n = 12 (standard chow–fed wild-type), 13 (HF diet–fed wild-type, standard chow– and HF diet–fed Gck+/–). (H) Insulin secretory index, defined as the ratio of insulin to glucose at 30 minutes after a glucose load. Values represent mean ± SEM. *P < 0.05; **P < 0.01.
Failure of compensatory β cell hyperplasia in Gck+/– mice on the HF diet.
Serum insulin levels are governed by a combination of the insulin secretory capacity of individual β cells and the number of β cells. In this study, we investigated islet mass first. Histologic analysis after 20 weeks on standard chow or HF diet revealed that the HF diet caused islet hyperplasia in the wild-type mice but, surprisingly, no islet hyperplasia in the Gck+/– mice (Figure 2A). Quantitative determinations of β cell and non–β cell mass showed that β cell mass increased by 110% in wild-type mice on the HF diet compared with those on standard chow, but that there was no significant increase in Gck+/– mice on the HF diet compared with those on standard chow (Figure 2B). After 4, 20, and 40 weeks on the HF diet, the wild-type mice showed 1.2-fold, 2.0-fold, and 10-fold increases, respectively, in β cell mass compared with wild-type mice on standard chow, whereas even after 40 weeks the Gck+/– mice showed only a 2-fold increase in β cell mass (Figure 2C). The number of cells per islet was significantly increased in wild-type mice on the HF diet compared with those on standard chow, but the difference between the 2 Gck+/– groups was not significant (Figure 2D). DNA content per islet was also significantly higher in wild-type mice than in Gck+/– mice on the HF diet (HF diet–fed wild-type, 39.1 ± 2.3 ng/islet, n = 6; HF diet–fed Gck+/–, 22.7 ± 1.9 ng/islet, n = 6; P < 0.001). To estimate the contribution of the size of individual β cells to the increase in β cell mass, we divided the β cell area by the number of β cell nuclei it contained. However, since the results showed little difference in β cell size between wild-type and Gck+/– mice on the HF diet (HF diet–fed wild-type, 155 ± 4 μm2, n = 101; HF diet–fed Gck+/–, 149 ± 4 μm2, n = 105; P = NS), the increased β cell mass in wild-type mice on the HF diet was attributed to an increase in the number of cells (hyperplasia) rather than to an increase in the volume of individual cells (hypertrophy).
Figure 2. Failure of compensatory β cell hyperplasia in HF diet–fed Gck+/– mice caused by decreased β cell replication rate.
(A) Histologic analysis of pancreatic islets of wild-type and Gck+/– mice after 20 weeks on standard chow or HF diet. Sections were double stained with anti-insulin antibody and a cocktail of anti-glucagon, anti-somatostatin, and anti-pancreatic polypeptide antibodies. Representative islets are shown. Red stain, β cells; brown stain, non–β cells. Scale bars: 100 μm. (B) Quantitation of β cell and non–β cell mass in wild-type and Gck+/– mice after 20 weeks on standard chow or HF diet. Areas of β or non–β cells (α, δ, and pancreatic polypeptide cells) are shown relative to total pancreas area (n = 4). (C) Changes in β cell mass on HF diet. Shown is β cell area relative to pancreas area (n = 4) after 4, 20, and 40 weeks on HF diet. (D) Number of cells in wild-type and Gck+/– mouse islets after 20 weeks on standard chow or HF diet (n = 6). (E and F) Replication rate of β cells, assayed (E) on the basis of BrdU incorporation after 20 weeks on standard chow or HF diet or (F) by PCNA staining after 20 weeks on HF diet. Results are shown as ratios of double-positive cells to insulin-positive cells (n = 4). Values represent mean ± SEM. *P < 0.05; **P < 0.01.
The number of β cells present is governed by a balance among β cell replication (increase in number of β cells by preexisting β cells), neogenesis (generation of β cells by non–β cells, such as acinar cells and duct cells), and apoptosis. We estimated β cell proliferation on the basis of BrdU incorporation and proliferating cell nuclear antigen (PCNA) staining. On the HF diet, there were significantly more insulin and BrdU double-positive cells in the wild-type mice than in the Gck+/– mice (Figure 2E), and similar results were obtained by PCNA staining (Figure 2F). Single-strand DNA analysis revealed no difference in the percentage of apoptotic cells in islets between wild-type and Gck+/– mice on the HF diet (HF diet–fed wild-type, 0.031% ± 0.026%, n = 86; HF diet–fed Gck+/–, 0.018% ± 0.018%, n = 33; P = NS). Fewer than 1 in 3,000 cells in the islets of both mouse groups were found to be apoptotic with an in situ cell death detection kit. Thus, in contrast to the wild-type mice, the failure of compensatory β cell hyperplasia in the Gck+/– mice on the HF diet was associated with a lack of compensatory increase in β cell proliferation.
Impaired glucose-stimulated insulin secretion associated with decreased glucose metabolism in the β cells of Gck+/– mice on the HF diet.
Next, we investigated glucose-stimulated insulin secretion (GSIS) by individual β cells. After 4 weeks, GSIS at 22.2 mM glucose normalized by cell number was lower in wild-type mice on the HF diet than in those fed standard chow (Figure 3A). Thus, the hyperinsulinemia in the wild-type mice on the HF diet can be explained by increased β cell mass, not by increased insulin secretion by individual β cells. GSIS at 22.2 mM glucose normalized by cell number was significantly lower in Gck+/– mice than in wild-type mice on both diets, a finding consistent with the results of our previous study (24). The hyperglycemia itself may have also affected the insulin secretory function of islets in the Gck+/– groups. GSIS at 22.2 mM glucose was lower in the islets of the HF diet groups than in those of the standard chow groups of both genotypes, and after 20 weeks it was significantly decreased in the HF diet groups compared with the standard chow groups (data not shown), a finding consistent with previous reports that prolonged exposure to FFA results in suppression of insulin release (25, 26). While islet hexokinase activity was similar in all 4 groups, Gck activity in the Gck+/– standard chow and HF diet groups was 30% and 25% lower than in the wild-type standard chow and HF diet groups, respectively, although the differences were not statistically significant (Figure 3B). When islets of essentially the same size were prepared and [U-14C]glucose oxidation was assayed in their mitochondria, glucose oxidation at 22.2 mM glucose was significantly lower in the HF diet than in standard chow groups of both phenotypes (Figure 3C). Importantly, after 20 weeks on the HF diet, glucose oxidation at 22.2 mM glucose did not significantly differ between wild-type and Gck+/– mice (Figure 3C), suggesting that normal glucose oxidation levels are not necessary for the compensatory increase in β cell mass, although they may be essential for GSIS.
Figure 3. Decreased insulin secretion and glucose oxidation in Gck+/– islets.
(A) Static incubation study of islets from wild-type and Gck+/– mice after 4 weeks on standard chow or HF diet. Static incubation of 10 islets/tube was performed at 37°C for 1 hour with various glucose concentrations after preincubation with a 2.8-mM glucose concentration for 20 minutes. Results are shown as pg insulin/cell/h (n = 4). (B) Gck and hexokinase (HK) activity of islets. Glucose phosphorylation activity was assessed in pancreatic islets from wild-type and Gck+/– mice after 20 weeks on standard chow or HF diet. Results are shown as mol/kg DNA/h (n = 16–20). (C) Glucose oxidation by pancreatic islets from wild-type and Gck+/– mice after 20 weeks on standard chow or HF diet. Results are shown as mol/kg DNA/h (n = 10). *P < 0.05; **P < 0.01.
Gene expression profiles of the islets of HF diet–fed mice.
We performed a DNA microarray analysis as a means of systematically examining the gene expression profiles of the islets. Of the 12,490 genes examined, 81 were overexpressed (by 3-fold or more; Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI17645DS1) and 63 were underexpressed (by 3-fold or more; Supplemental Table 2) in the islets of Gck+/– mice on the HF diet compared with the islets of wild-type mice on the HF diet. Interestingly, markedly lower expression of Irs2 (25-fold decrease, the greatest decrease in expression level of the genes examined), Pdpk1 (3-fold decrease), and Hgf (3.1-fold decrease) was observed in Gck+/– islets compared with those of wild-type mice, yet there were no differences in expression of Insr, Irs1, Irs3, Pik3r1, Pik3r2, Pik3ca, or Akt1 (Table 1). Expression of Igf1r (2.4-fold decrease) and Prlr (2.6-fold decrease) was also modestly lower in Gck+/– islets than in wild-type islets. By contrast, there was no difference between the 2 HF diet–fed groups in expression of apoptosis-related genes such as Casp3 and Bad in the islets. RT-PCR analysis confirmed the upregulation of Irs2, Igf1r, and Prlr expression in the islets of wild-type mice on the HF diet compared with those on standard chow as well as reduced expression in the islets of Gck+/– mice on the HF diet compared with those of HF diet–fed wild-type mice (Figure 4A). Quantitative PCR analysis, which amplified another region of the Irs2 and Igf1r genes, revealed that expression of Irs2 and Igf1r in the islets of Gck+/– mice on the HF diet was 60.6% (P < 0.01 versus wild-type mice) and 76.4%, respectively, that of their expression level in the islets of wild-type mice on the HF diet.
Table 1 .
Changes in gene expression levels in islets based on DNA microarray analysis
Figure 4. Changes in gene expression levels in the islets of Gck+/– mice on the HF diet.
(A) RT-PCR analysis of Irs1, Irs2, Igf1r, Prlr, Ipf1, and Arbp (36B4), shown as a control. Islets were isolated from wild-type or Gck+/– mice after 20 weeks on standard chow or HF diet. Experiments were replicated at least 3 times, and typical images are shown. (B) Western blot analysis of Irs2, Igf1r, Insr, Ipf1, and Akt1. Islets were isolated from wild-type or Gck+/– mice after 20 weeks on standard chow or HF diet, Irs1–/– mice, and Irs2–/– mice on standard chow (n = 3). Equal amounts of lysates (20 μg) were blotted with the antibody indicated. Quantitative determination of the β cell mass of islets less than 250 μm in diameter revealed the values in the 4 mouse groups to be indistinguishable (standard chow–fed wild-type, 0.81% ± 0.03%; HF diet–fed wild-type, 0.85% ± 0.03%; standard chow–fed Gck+/–, 0.82% ± 0.02%; HF diet–fed Gck+/–, 0.84% ± 0.02%). (C) Each expression level was quantified (n = 4–6). *P < 0.05; **P < 0.01.
Next, we examined the protein levels by Western blotting. We prepared islets (less than 250 μm in diameter) from mice after 20 weeks on each diet. The results confirmed upregulation of Irs2 and Igf1r expression in the islets of wild-type mice on the HF diet compared with those on standard chow as well as reduced expression in the islets of Gck+/– mice on the HF diet compared with those of wild-type mice on the HF diet (Figure 4, B and C). Interestingly, expression of Insr was significantly increased in the HF diet–fed groups compared with standard chow–fed groups (Figure 4, B and C), although there were no differences in expression of Akt1 among the 4 groups. Ipf1 expression was indistinguishable between the islets of Gck+/– and wild-type mice on standard chow, but the protein level was slightly, but significantly, lower in the islets of Gck+/– mice on the HF diet than in those of wild-type mice on the HF diet (Figure 4, B and C). Immunostaining revealed clearly lower Ipf1 nuclear expression in the β cells of Gck+/– mice on the HF diet than in those of wild-type mice on the HF diet (Supplemental Figure 1).
In summary, DNA microarray and RNA and protein analyses revealed upregulation of Irs2 and Igf1r expression in the islets of wild-type mice on the HF diet compared with those fed standard chow as well as reduced expression in the islets of Gck+/– mice on the HF diet compared with those of HF diet–fed wild-type mice.
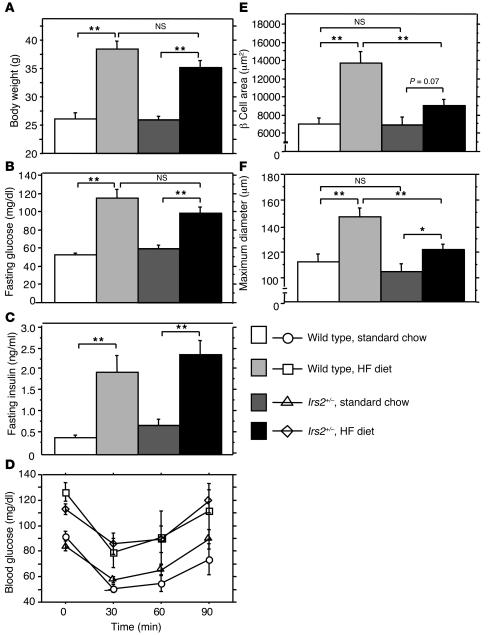
Insufficient increase in β cell mass in Irs2+/– mice on the HF diet.
Next we investigated the role of Irs2 in the regulation of β cell mass in mice on the HF diet. After 10 weeks on the HF diet, Irs2+/– mice exhibited increases in body weight, blood glucose, serum insulin, and insulin resistance similar to those of HF diet–fed wild-type mice (Figure 5, A–D). The Irs2+/– mice had β cell area and maximum islet diameter similar to those of wild-type mice fed standard chow, but the increases were significantly smaller than those observed in HF diet–fed wild-type mice (Figure 5, E and F). After only 5 weeks on the HF diet, Irs2–/– mice showed marked aggravation of glucose intolerance (27). While the mean of the maximum islet diameter was 14% greater in wild-type mice on the HF diet than in those on standard chow, a difference that was significant, there was no such increase in the Irs2–/– mice on the HF diet compared with those on standard chow (data not shown). These results support a role for Irs2 in the increase in β cell mass on the HF diet.
Figure 5. Insufficient β cell hyperplasia in Irs2+/– mice on the HF diet.
(A–C) Body weight (A), fasting blood glucose (B), and serum insulin (C) values of wild-type and Irs2+/– mice after 10 weeks on standard chow or HF diet (n = 5–8). (D) Insulin tolerance in wild-type and Irs2+/– mice after 10 weeks on standard chow or HF diet (n = 4–8). (E and F) Area of β cells in each islet (E) and maximum islet diameter (F) in wild-type and Irs2+/– mice after 10 weeks on standard chow or HF diet. We examined 100–150 islets from 3 animals per group. Values represent mean ± SEM. *P < 0.05; **P < 0.01.
Transgenic rescue by crossing Gck+/– mice with β cell Irs2 transgenic mice.
To directly test our hypothesis that reduction of Irs2 explains the impaired β cell hyperplasia in Gck+/– mice on HF diet, we crossed Gck+/– mice with β cell Irs2 transgenic (βIrs2Tg) mice, which express Irs2 in β cells under the control of the rat insulin promoter, and generated 2 lines of βIrs2Tg mice (Supplemental Figure 2, A and B). While the β cells of βIrs2Tg6 mice expressed a low level of Irs2 (2.2-fold upregulation compared with wild-type littermates), the β cells of βIrs2Tg12 mice expressed a high level of Irs2 (>17-fold upregulation). We chose to use the βIrs2Tg6 line because the 2.2-fold upregulation of Irs2 in βIrs2Tg6 mice was comparable to the Irs2 expression level in wild-type mice on the HF diet. We crossed βIrs2Tg6 mice with Gck+/– mice and obtained 4 genotypes: wild-type, βIrs2Tg, Gck+/–, and βIrs2TgGck+/–. Only male mice were used in the experiments. After 20 weeks on the HF diet, the fold change in Irs2 protein expression compared with Gck+/– mice was 3.5 ± 0.2 for wild-type, 4.6 ± 0.5 for βIrs2Tg, 1.0 ± 0.1 for Gck+/–, and 3.1 ± 0.6 for βIrs2TgGck+/– mice (Supplemental Figure 2, C and D). These results can be explained by the fact that HF diet upregulated endogenous Irs2 expression in the islets of wild-type and βIrs2Tg mice, but not the islets of Gck+/– or βIrs2TgGck+/– mice. After 4 weeks on the HF diet, the glucose tolerance of βIrs2Tg mice was similar to that of wild-type mice, but the βIrs2TgGck+/– mice had better glucose tolerance than did Gck+/– mice (data not shown). After 20 weeks on the HF diet, the βIrs2Tg mice had glucose tolerance similar to that of wild-type mice, but the βIrs2TgGck+/– mice had better glucose tolerance and higher serum insulin levels than did Gck+/– mice (Figure 6, A and B). Thus, overexpression of Irs2 partially prevented diabetes in Gck+/– mice on the HF diet.
Figure 6. Transgenic rescue by crossing Gck+/– mice with βIrs2Tg mice.
(A and B) Glucose tolerance in wild-type, Gck+/–, βIrs2Tg, and βIrs2TgGck+/– mice after 20 weeks on HF diet. (A) Plasma glucose levels. (B) Serum insulin levels. n = 31 (wild-type), 20 (βIrs2Tg), 35 (Gck+/–), 16 (βIrs2TgGck+/–). *P < 0.05, Gck+/– versus βIrs2TgGck+/–. (C) Histologic analysis of wild-type, Gck+/–, βIrs2Tg, and βIrs2TgGck+/– mouse islets after 20 weeks on HF diet. Representative pancreatic islets are shown. Top panels show insulin staining; bottom panels show BrdU staining. Scale bars: 100 μm. Original magnification, ×100 (top panels); ×400 (bottom panels). (D) Area of β cells in each islet after 20 weeks on HF diet. We examined 100–150 islets from 3 animals per group. (E) Replication rate of β cells, assayed on the basis of BrdU incorporation after 20 weeks on HF diet. Results are shown as ratios of insulin and BrdU double-positive cells to insulin-positive cells (n = 4). (F) Static incubation study of islets after 20 weeks on the HF diet. Static incubation of 10 islets/tube was performed at 37°C for 1 hour with various glucose concentrations after preincubation with a 2.8-mM glucose concentration for 20 minutes. Results are shown as pg insulin/cell/h (n = 4). Values represent mean ± SEM. *P < 0.05; **P < 0.01.
Figure 6C shows representative insulin staining of the pancreas after 20 weeks on the HF diet. Measurement of the β cell area showed that it was significantly increased in βIrs2TgGck+/– mice compared with Gck+/– mice (Figure 6D), and consistent with this finding, there were significantly more insulin and BrdU double-positive cells in βIrs2TgGck+/– mice than in Gck+/– mice (Figure 6, C and E). Next we isolated islets from the 4 mouse groups after 20 weeks on the HF diet and carried out static incubation experiments. GSIS at 11.1 mM glucose was not restored in βIrs2TgGck+/– mice compared with Gck+/– mice (Figure 6F), indicating that overexpression of Irs2 failed to restore β cell function in Gck+/– mice.
Mechanisms of Irs2 upregulation in the β cells of HF diet–fed mice.
We examined known regulators of Irs2 expression, such as cAMP, and subsequent phosphorylation of cAMP response element–binding protein (CREB) (28) and FoxO1 (29). Although the cAMP content of the islets was unaffected by the HF diet (data not shown) and the levels of expression of cAMP-responsive genes — including Creb1, Glp1r, Prkar1a, Prkar1b, Prkar2a, Prkaca, Prkacb, and Lasp1 — were unaltered (Table 1), Ser133 phosphorylation of CREB was impaired in Gck+/– mice compared with wild-type mice (Figure 7, A and B). FoxO1 is an activator of Irs2 in liver, because the Irs2 promoter is activated by FoxO1 through an insulin response element (29), but it has also been characterized as a key distal mediator of insulin signaling, because FoxO1 haploinsufficiency reverses β cell failure in Irs2–/– mice (30). Immunohistochemical analysis revealed that FoxO1 was more localized in the nuclei of β cells of Gck+/– mice on the HF diet than in those of wild-type mice on the HF diet (Figure 8A). In fact, the ratio of nuclear FoxO1-positive cells to the total number of islet cells was significantly higher in Gck+/– mice on the HF diet than in wild-type mice on the HF diet (Figure 8B). Overexpression of Irs2 in β cells decreased the number of nuclear FoxO1-positive cells in the Gck+/– mice on the HF diet, indicating that upregulation of Irs2 in β cells stimulated FoxO1 nuclear exclusion (Figure 8, A and B).
Figure 7. Impaired Ser133 phosphorylation of CREB in Gck+/– mice on HF diet.
(A) Western blot assay of Ser133-phosphorylated CREB (p-CREB) and total CREB levels in islets from wild-type and Gck+/– mice after 20 weeks on standard chow or HF diet. (B) Quantitation of Ser133 phosphorylation of CREB in wild-type and Gck+/– mice after 20 weeks on standard chow or HF diet. Results are shown as proportions of the intensity of the Ser133-phosphorylated CREB band to that of the total CREB band (n = 5). Ser133 phosphorylation of CREB was significantly impaired in Gck+/– mice compared with wild-type mice on the HF diet. Values represent mean ± SEM. *P < 0.05.
Figure 8. Nuclear FoxO1-positive cells increased in Gck+/– mice on the HF diet compared with wild-type mice on the HF diet, and overexpression of Irs2 in β cells decreased nuclear FoxO1-positive cells in Gck+/– mice on the HF diet.
(A) Immunohistochemical analysis of FoxO1 in islets from wild-type mice, βIrs2Tg mice, Gck+/– mice, and βIrs2TgGck+/– mice after 20 weeks on the HF diet. Representative islets are shown. FoxO1-positive cells are stained brown. Original magnification, ×600. (B) Ratio of the number of nuclear FoxO1-positive cells to the total number of islet cells. n = 43 (wild-type, Gck+/–), 23 (βIrs2Tg), 48 (βIrs2TgGck+/–). Values represent mean ± SEM. **P < 0.01.
The above findings, together with the fact that cAMP and calcium pathways trigger CREB Ser133 phosphorylation, thereby upregulating Irs2 (28), suggest that impaired CREB Ser133 phosphorylation is a plausible explanation for the insufficient upregulation of Irs2 in Gck+/– mice on the HF diet. The significance of the decreased FoxO1 nuclear exclusion in Gck+/– mice on the HF diet are discussed below.
Discussion
Gck is known to function as a glucose sensor in insulin secretion by pancreatic β cells (4), but to our knowledge, its involvement in the regulation of β cell mass had previously not been recognized. In this paper, we report 4 findings, which we believe to be novel, that link Gck to β cell mass. First, we found that wild-type mice on a HF diet showed marked compensatory β cell hyperplasia associated with increased replication of β cells, whereas Gck+/– mice failed to show as much β cell hyperplasia despite showing a similar degree of insulin resistance (Figure 2). The finding that haploinsufficiency of Gck led to insufficient β cell hyperplasia on the HF diet suggests a critical requirement for Gck, not only for GSIS, but for β cell hyperplasia in response to HF diet–induced insulin resistance to protect against diabetes. Although glucose itself has been recognized to be a nutrient for β cells, to our knowledge it was previously unknown whether glucose metabolism is crucial for β cell growth (7). The results of this study in regard to the role of glucose metabolism and Gck in the regulation of β cell mass clearly demonstrate that Gck is associated with β cell mass and proliferation, raising the possibility that other enzymes involved in glucose metabolism may have a similar effect on β cell growth. Second, we found that on the HF diet, expression of Irs2 and Igf1r was upregulated in wild-type islets but markedly lower in the islets of Gck+/– mice (Table 1 and Figure 4, A–C), showing that Gck is required for the coordinated upregulation of Irs2 and Igf1r in the islets of HF diet–fed mice. Secondary effects of chronic hyperglycemia may also have contributed to the decreased Irs2 and Igf1r expression in the islets of Gck+/– mice on the HF diet. It should be noted, however, that wild-type mice after 20 weeks on the HF diet and Gck+/– mice on standard chow had similar glucose tolerance (Figure 1F) and that after another 20 weeks the HF diet–fed wild-type mice showed a greater increase in β cell mass, whereas even after 40 weeks on the HF diet the Gck+/– mice showed only a small increase (Figure 2C). These results indicate that chronic hyperglycemia alone cannot fully explain the insufficient β cell hyperplasia of Gck+/– mice on the HF diet. Third, in a previous study we showed a lack of β cell hyperplasia in response to genetically determined insulin resistance in Irs2–/– mice (11), and in the present study we demonstrated that haploinsufficiency of Irs2 led to insufficient β cell hyperplasia on the HF diet (Figure 5, E and F). Fourth, we found that overexpression of Irs2 partially prevented diabetes in Gck+/– mice on the HF diet. It should be noted, however, that the slight improvement in glucose tolerance can be explained by the fact that β cell mass, not β cell function, was restored in βIrs2TgGck+/– mice compared with Gck+/– mice on the HF diet (Figure 6, C–E). These results provide genetic evidence that Irs2 is critically involved in β cell hyperplasia on the HF diet. Based on these findings, we propose that both Gck and Irs2 are critical requirements for β cell hyperplasia in response to HF diet–induced insulin resistance.
What is the mechanism of Irs2 upregulation on the HF diet? We previously reported that the increase in intracellular calcium concentration in response to glucose was impaired in islets of Gck+/– mice (ref. 3 and our unpublished observations). In the present study we noted the impaired CREB Ser133 phosphorylation in Gck+/– mice on the HF diet. Taken together with the fact that cAMP and calcium pathways trigger CREB Ser133 phosphorylation, thereby upregulating Irs2 (28), our present results suggest that the impaired calcium signaling caused by haploinsufficiency of Gck in combination with HF diet–induced insulin resistance leads to the impaired Ser133 phosphorylation of CREB. Further study is needed to test this hypothesis.
What, then, is the molecular link between Irs2 and β cell proliferation? We noted the decreased FoxO1 nuclear exclusion in Gck+/– mice on the HF diet compared with wild-type mice on the HF diet (Figure 8, A and B). This finding, together with previous reports of a role of FoxO1 downstream of Irs2 in linking insulin signaling to Ipf1 regulation of β cells and compensation to insulin resistance (30, 31), suggests that decreased FoxO1 nuclear exclusion contributes to the insufficient proliferative response of existing β cells to insulin resistance in Gck+/– mice on the HF diet, and the fact that overexpression of Irs2 in β cells stimulated FoxO1 nuclear exclusion in wild-type and Gck+/– mice (Figure 8, A and B) is consistent with this hypothesis. Moreover, we noted lower Ipf1 nuclear expression in the β cells of Gck+/– mice on the HF diet than in those of wild-type mice on the HF diet (Supplemental Figure 1), which can be explained by the decreased FoxO1 nuclear exclusion in Gck+/– mice on the HF diet, as previously demonstrated in Irs2–/– mice (30). However, because haploinsufficiency of Ipf1 led to impaired β cell function, but not to decreased β cell mass (32), whether decreased Ipf1 nuclear expression is involved in the decreased β cell hyperplasia in Gck+/– mice on the HF diet remains unresolved. To determine whether Ipf1 is involved in the regulation of β cell mass in Gck+/– mice, we are now investigating the phenotypes obtained by crossing Gck+/– mice with β cell–specific Ipf1 transgenic mice.
Recently, β cell–specific ablation of Pdpk1 has been shown to induce diabetes as a result of loss of β cell mass (33); in the present study, expression of Pdpk1 actually decreased in HF diet–fed Gck+/– mice compared with wild-type mice (Table 1). Thus, inadequate activation of Pdpk1 downstream of Irs2 signaling may also play a role in the insufficient compensatory β cell hyperplasia in Gck+/– mice on the HF diet. Interestingly, while expression of cyclin D1, cyclin D3, CDK4, and cyclin-dependent kinase inhibitors including p21 and p27 Kip1 was unaltered in the islets of Gck+/– mice on the HF diet compared with those of wild-type mice on the HF diet, expression of cyclin D2 was downregulated (5.8-fold decrease) and expression of p15 inhibitor was slightly upregulated (1.2-fold increase). The roles of these cell cycle–related molecules in β cell proliferation downstream of Irs2 signaling should be examined in a future study.
What is the relationship between glucose metabolism and β cell proliferation? It has been established that β cell function, including GSIS, can be explained by glucose metabolism (4). Glucose oxidation was decreased to a similar degree in both wild-type and Gck+/– mice on the HF diet (Figure 3C). Although the wild-type mice showed marked compensatory β cell hyperplasia, the Gck+/– mice failed to show such β cell hyperplasia, demonstrating that glucose oxidation is not directly linked to β cell mass. Moreover, Gck activity in the wild-type mice was not increased on the HF diet compared with standard chow (Figure 3B). From a biochemical standpoint, “classical” glucose metabolism cannot explain β cell proliferation on the HF diet, and some additional mechanism is required. It may be premature, however, to rule out an involvement of glucose metabolism in HF diet–induced β cell hyperplasia based on the studies of glucose oxidation in isolated islets, because there are no doubt important glucose signals left to be discovered, as evidenced from the pursuit of the mechanisms of KATP-independent GSIS. Although β cell function and β cell growth have previously been thought to be regulated differently, the results of the present study indicate that both may be regulated in part by a coordinated or common mechanism.
What is the relevance of our results to clinical practice in human diabetes? Relatively common mutations of the Gck gene have been reported in MODY patients (2, 34), but since they are not restricted to the β cell–specific isoform, the diabetic phenotype of these patients is due to a combination of defects in insulin secretion and glucose uptake by the liver. By contrast, a SNP in the promoter region of the Gck gene has been reported to be associated with reduced β cell function in Japanese-American men (35). The results of our study are somewhat surprising, as the majority of the known clinical cases of MODY have been mild cases of diabetes (2, 34). However, the severity of diabetes is also known to differ among patients with the same mutation, and the differences may be attributable to peripheral insulin resistance associated with obesity and/or environmental factors (34, 36). Because a HF diet is one of the pivotal factors in the etiology of insulin resistance and obesity, our results should be relevant to the diversity in diabetes severity among MODY patients. Furthermore, since type 2 diabetes patients with decreased insulin secretion have been shown to have reduced β cell mass (37), Gck+/– mice on a HF diet should serve as a good animal model to better understand the relationship between decreased insulin secretion and decreased β cell mass in type 2 diabetes. Gck activation via small-molecule Gck activators in combination with increased cAMP production via glucagon-like peptide 1 derivatives may be a powerful strategy for the treatment of the decreased insulin secretion and decreased β cell mass in type 2 diabetes. In conclusion, the results of our study support the concept that Gck regulates β cell mass as well as β cell function. Irs2 was found to be involved in Gck-mediated β cell hyperplasia in HF diet–fed mice. Identification of the mechanism linking Gck and Irs2 should lead to novel therapeutic strategies that will increase β cell mass to compensate for HF diet–induced insulin resistance and may increase the amount of islets (β cells) for islet transplantation.
Methods
Animals.
Gck+/– mice (129/Sv, ICR, and C57BL/6J hybrid background) were generated as described previously (3). Because of the heterogeneous genetic background of the mice, male offspring derived from Gck+/– intercrosses were analyzed in this study. The Gck+/– and wild-type mice were fed standard chow until 8 weeks of age and were then given free access to either standard chow or a HF diet. The animal care and procedures of the experiments were approved by the Animal Care Committee of the University of Tokyo. Animals were maintained by means of standard animal care procedures based on the institutional guidelines. Irs1–/– and Irs2–/– mice (CBA and C57BL/6J hybrid background) were generated as described previously (8, 11). The βIrs2Tg mouse lineage was established by fusing the 0.74-kb rat insulin II promoter to a mouse Irs2 cDNA (4 kb), microinjecting the purified NotI fragment into the pronucleus of eggs of fertilized C57BL/6J mice (CLEA Japan Inc.), and then crossing F1 offspring with C57BL/6J mice (Supplemental Figure 2, A and B). Male offspring littermates derived from crosses between male Gck+/– mice and female βIrs2Tg mice were analyzed in the Irs2 rescue experiments (Supplemental Figure 2, C and D).
Measurement of serum and islet parameters.
Glucose, insulin, triglyceride, and FFA levels were determined with a Glutest Pro kit (SANWA KAGAKU KENKYUSHO CO. LTD), an insulin kit (Biotrak; Amersham Biosciences), and L-type TG M and NEFA C-test kits (Wako), respectively.
Diet protocol.
The composition of the standard chow (CLEA Rodent Diet CE-2; CLEA Japan Inc.) was 50.7% (wt/wt) carbohydrate, 4.6% fat, 25.2% protein, 4.4% dietary fiber, 6.5% crude ash, 3.6% mineral mixture, 1% vitamin mixture, and 4% moisture. The HF diet study was carried out according to previously described methods (38, 39). The composition of the HF diet was 32% safflower oil, 33.1% casein, 17.6% sucrose, 5.6% cellulose, 9.8% mineral mixture, 1.4% vitamin mixture, and 0.5% DL-methionine.
In vivo glucose homeostasis.
Glucose and insulin tolerance tests were performed as described previously (39, 40).
Histologic and immunohistochemical analysis and determination of adipocyte size.
Adipose tissue was fixed with formaldehyde, and 10-μm sections were cut, mounted on glass slides, and stained with hematoxylin and eosin. White adipocyte areas were measured in 300 or more cells per mouse in each group, as described previously (39).
Immunohistochemical analysis of the endocrine pancreas and estimation of β cell and non–β cell mass and individual β cell size.
Isolated pancreata were immersion-fixed in Bouin’s solution at 4°C overnight. Tissue was then routinely processed for paraffin embedding, and 2-μm sections were cut and mounted on glass slides. The sections were double immunostained with guinea pig anti-porcine insulin antibody (diluted 1:200) and a cocktail composed of rabbit anti-porcine glucagon (diluted 1:200), rabbit anti-human somatostatin (diluted 1:800), and rabbit anti-human pancreatic polypeptide (diluted 1:800) antibodies (all from Dako). Images of pancreatic tissue, islet β cells, and islet non–β cells were captured on a computer through a microscope connected to a charge-coupled device camera (all from Olympus). The area of the β cells and non–β cells relative to the total area of pancreatic tissue was calculated with WinROOF software (version 5.0; Mitani Corp.) as described previously (41). More than 20 pancreatic sections from each animal, including representative sections of the head, body, and tail of the pancreas, were analyzed, and approximately 100 islets per mouse were counted in each group. Adjacent nonoverlapping fields were analyzed to obtain a true representation of average islet/β cell distribution throughout the pancreas. Individual β cell size was estimated by dividing the β cell area by the number of β cell nuclei in the area covered, as described previously (21). Maximum islet diameter was also calculated with WinROOF software. Immunostaining with anti-Ipf1 antibody (42) was performed as described previously (43). FoxO1 was immunohistochemically analyzed with anti-FoxO1 antibody (Cell Signaling Technology).
BrdU incorporation analysis and PCNA staining.
BrdU incorporation was analyzed as described previously (44). In brief, BrdU (100 mg/kg in saline; Sigma-Aldrich) was intraperitoneally injected, and the pancreas was removed 6 hours later. The sections were double immunostained with anti-BrdU antibody (diluted 1:200; Dako) and a cocktail of anti-glucagon, anti-somatostatin, and anti-pancreatic polypeptide antibodies. BrdU-positive β cells were quantitatively assessed as a proportion of all β cells by counting the cells in approximately 50 islets per mouse. Sections were immunostained for PCNA with mouse anti-PCNA antibody (diluted 1:10; Progen Biotechnik) at 4°C for 48 hours.
Detection of apoptotic cells.
Single-strand DNA analysis in islets was performed as described previously (45). Apoptotic cells were also detected in deparaffinized pancreatic sections by using an in situ cell death detection kit (Roche Diagnostics) according to the manufacturer’s recommendations.
Islet isolation.
For metabolic analysis, islets were isolated with collagenase as described previously (46). For preparation for RNA or protein, islets were isolated by using liberase RI (Roche Diagnostics) according to the manufacturer’s instructions.
Analysis of insulin secretion and determination of glucose-phosphorylating activity, glucose oxidation, and cAMP content.
Although the cellular composition of the islets may be affected by the dietary regimen, we used islets of comparable size (less than 250 μm in diameter) to assess islet insulin secretion and glucose metabolism, because quantitative determination of β cell mass in islets less than 250 μm in diameter yielded values in the 4 mouse groups that were indistinguishable. Insulin secretion by islets was analyzed as described previously (46). Glucose phosphorylation by hexokinase and Gck was assayed fluorometrically as described previously (3, 47). Hexokinase activity was measured at a glucose concentration of 0.5 mM, and the Gck activity was estimated as the difference between activity at 0.5 mM glucose and activity at 50 mM glucose. [U-14C]glucose oxidation in mitochondria was assayed by measuring [14C]CO2 production as described previously (46). To extract cAMP for measurements, islet cells were disrupted by sonication for 5 seconds in 500 μl 95% ethanol at 4°C, vortexed vigorously, and centrifuged at 18,000 g for 30 minutes at 4°C. After removing the supernatant and evaporating to dryness, the samples were redissolved in sodium acetate buffer (0.05 mol/l, pH 6.2), and cAMP levels were determined with an enzyme-linked immunosorbent assay kit (cAMP Biotrak Enzymeimmunoassay System; Amersham Biosciences) in 96-well plates on a spectrophotometer at 450 nm according to the manufacturer’s instructions.
Microarray analysis of mRNA levels in isolated islets.
Isolated islets were cultured overnight in RPMI 1640 medium containing 11.1 mM glucose (Sigma-Aldrich) supplemented with 10% FBS, 0.075 g/l penicillin (Sigma-Aldrich), and 0.1 g/l streptomycin (Sigma-Aldrich). Total RNA was isolated with the RNeasy Mini Kit (Qiagen) and used as starting material for cDNA preparation. RNA was prepared from 7 mice of identical genotype. The first- and second-strand cDNA synthesis, array hybridization, and scanning were performed as described previously (48). In brief, RNA amplification was started with 5 μg of total islet RNA. Double-stranded cDNA was synthesized with the SuperScript Choice system (Gibco) and a T7-(dT) 24 Primer (Amersham Pharmacia Biotech). In vitro transcription was performed to produce biotin-labeled cRNA by using a BioArray HighYield RNA Transcript Labeling Kit (Affymetrix) according to the manufacturer’s instructions. cRNA was linearly amplified approximately 40-fold with T7 polymerase by using half of the double-stranded cDNA that was synthesized. The readings obtained by quantitative scanning were analyzed with Affymetrix Gene Expression Analysis software. For expression change calls, we used the comparative analysis (Wilcoxon signed rank test–based analysis) program in Microarray Suite version 5.0 as described previously (49). The analysis was performed with the default parameters, where the P value of significant difference was below 0.0025.
Immunoblotting.
The polyclonal anti-Irs1, anti-Irs2, and anti-p85 antibodies were purchased from Upstate USA Inc. The polyclonal anti-Insr antibody, and anti–Igf1rβ antibody were purchased from Santa Cruz Biotechnology Inc. The anti-Akt antibody, anti-Igf1rα antibody, anti-CREB antibody, and phospho-CREB (Ser133) antibody were purchased from Cell Signaling Technology Inc. The polyclonal anti-Ipf1 antibody (42) was provided by Y. Kajimoto (Osaka University, Suita, Japan). Islets were sonicated in ice-cold buffer A (25 mM Tris-HCl, pH 7.4, 10 mM Na3VO4, 10 mM NaPPi, 100 mM NaF, 10 mM EDTA, 10 mM EGTA, and 1 mM phenylmethylsulfonyl fluoride) with an ultrasonic sonicator. Samples were separated by SDS-polyacrylamide gel electrophoresis, and immunodetection was performed with an ECL kit (Amersham Biosciences). Protein was prepared from more than 100 islets pooled from several mice of identical genotype, and 20 μg samples of proteins were applied to the gel.
Statistics.
Results are expressed as mean ± SEM. Statistical analysis was performed using the StatView software system (version 4.5; Abacus). Differences between 2 groups were analyzed for statistical significance by Student’s t test for unpaired comparisons. Individual comparisons among more than 2 groups were assessed with the post-hoc Fisher’s pairwise least-significant-difference test. A P value less than 0.05 was considered to be statistically significant.
Supplementary Material
Acknowledgments
We dedicate this paper to K. Komeda, who devoted his life to studying the pathophysiology of diabetes with animal models. We thank Y. Kajimoto for the generous gift of anti-Ipf1 antibody and Y. Meguro, E. Yoshida, A. Nagano, Y. Muto, M. Nakashima, N. Kowatari, and H. Chiyonobu for their excellent technical assistance and animal care. This work was supported by a grant for Life & Socio-Medical Science from the Kanae Foundation, a grant by the Tanabe Medical Frontier Conference, a Grant-in-Aid for Scientific Research in Priority Areas (B) 15390285 and (B) 16390263 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant for the 2006 Strategic Research Project of Yokohama City University (to Y. Terauchi) as well as a Grant-in-Aid for Creative Scientific Research (10NP0201) from the Japan Society for the Promotion of Science, a Grant-in-Aid for Scientific Research in Priority Areas (C), a Grant-in-Aid for the Development of Innovative Technology from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Health Science Research Grants (Research on the Human Genome and Gene Therapy) from the Ministry of Health and Welfare, a grant from the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan, and a Grant-in-Aid (1-2000-231 and 1-2003-746) from the Juvenile Diabetes Research Foundation (to T. Kadowaki).
Footnotes
Nonstandard abbreviations used: CREB, cAMP response element–binding protein; Gck, glucokinase; GSIS, glucose-stimulated insulin secretion; HF, high-fat; Igf1r, Igf1 receptor; Insr, insulin receptor; Ipf1, insulin promoter factor 1; IRS, insulin receptor substrate; βIrs2Tg, β cell Irs2 transgenic (mice); MODY, maturity-onset diabetes of the young; PCNA, proliferating cell nuclear antigen.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:246–257 (2007). doi:10.1172/JCI17645
Yasuo Terauchi, Iseki Takamoto, and Naoto Kubota contributed equally to this work.
Kajuro Komeda is deceased.
See the related Commentary beginning on page 81.
References
- 1.Matschinsky F.M., Ellerman J.E. Metabolism of glucose in the islets of Langerhans. J. Biol. Chem. 1968;243:2730–2736. [PubMed] [Google Scholar]
- 2.Velho G., et al. Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia. 1997;40:217–224. doi: 10.1007/s001250050666. [DOI] [PubMed] [Google Scholar]
- 3.Terauchi Y., et al. Pancreatic β-cell-specific targeted disruption of glucokinase gene. J. Biol. Chem. 1995;270:30253–30256. doi: 10.1074/jbc.270.51.30253. [DOI] [PubMed] [Google Scholar]
- 4.Matschinsky F.M. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 5.Taylor S.I., Accili D., Imai Y. Insulin resistance or insulin deficiency: which is the primary cause of NIDDM? Diabetes. 1994;43:735–740. doi: 10.2337/diab.43.6.735. [DOI] [PubMed] [Google Scholar]
- 6.Polonsky K.S., Sturis J., Bell G.I. Non-insulin-dependent diabetes mellitus-A genetically programmed failure of the beta cell to compensate for insulin resistance. N. Engl. J. Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 7.Bonner-Weir S. Postnatal pancreatic β cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 8.Tamemoto H., et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 9.Araki E., et al. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 10.Withers D.J., et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 11.Kubota N., et al. Disruption of insulin receptor substrate-2 causes type 2 diabetes due to liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 12.Shulman G.I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamauchi T., et al. Replenishment of the fat-derived hormone adiponectin reverses insulin resistance in lipoatrophic diabetes and type 2 diabetes. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 14.Weisberg S.P., et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni R.N. New insights into the roles of insulin/IGF-I in the development and maintenance of β-cell mass. Rev. Endocr. Metab. Disord. 2005;6:199–210. doi: 10.1007/s11154-005-3051-y. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes C.J. IGF-I and GH post-receptor signaling mechanisms for pancreatic β-cell replication. J. Mol. Endocrinol. 2000;24:303–311. doi: 10.1677/jme.0.0240303. [DOI] [PubMed] [Google Scholar]
- 17.Ahlgren U., Jonsson J., Jonsson L., Simu K., Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. . 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakae J., et al. Regulation of insulin action of pancreatic β-cell function by mutant alleles of the forkhead transcription factor Foxo1. Nat. Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 19.Ueki K., et al. Total insulin and IGF-I resistance in pancreatic β cells cause overt diabetes. Nat. Genet. 2006;38:583–588. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]
- 20.Tuttle R.L., et al. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 21.Bernal-Mizrachi E., Wen W., Stahlhut S., Welling C.M., Permutt M.A. Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J. Clin. Invest. 2001;108:1631–1638. doi: 10.1172/JCI200113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freemark M., et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Ocana A., et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa T., et al. Analysis of pancreatic β cell in the mouse with targeted disruption of the pancreatic β cell-specific glucokinase gene. Biochem. Biophys. Res. Commun. 1996;229:460–465. doi: 10.1006/bbrc.1996.1826. [DOI] [PubMed] [Google Scholar]
- 25.Unger R.H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 26.Mason T.M., et al. Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes. 1999;48:524–530. doi: 10.2337/diabetes.48.3.524. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki R., et al. Pdx1 expression in Irs2 deficient mouse β-cells is regulated in a strain-dependent manner. J. Biol. Chem. 2003;278:43691–43698. doi: 10.1074/jbc.M307004200. [DOI] [PubMed] [Google Scholar]
- 28.Jhala U.S., et al. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ide T., et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat. Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T., et al. The forkhead transcription factor FoxO1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J. Clin. Invest. 2002;110:1839–1847. doi: 10.1172/JCI200216857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamaoto H., et al. Role of the forkhead protein FoxO1 in β cell proliferation to insulin resistance. J. Clin. Invest. 2006;116:775–782. doi: 10.1172/JCI24967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih D.Q., et al. Profound defects in pancreatic β-cell function in mice with combined heterozygous mutations in Pdx-1, Hnf-1α, and Hnf-3β. Proc. Natl. Acad. Sci. U. S. A. 2002;99:3818–3823. doi: 10.1073/pnas.062605899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto N., et al. Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of beta cell mass. Nat. Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- 34.Fajans S.S., Bell G.I., Polonsky K.S. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N. Engl. J. Med. . 2001;345:971–980. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- 35.Stone L.M., Kahn S.E., Fujimoto W.Y., Deeb S.S., Porte D. A variation at position -30 of the beta-cell glucokinase gene promoter is associated with reduced beta-cell function in middle-aged Japanese-American men. Diabetes. 1996;45:422–428. doi: 10.2337/diab.45.4.422. [DOI] [PubMed] [Google Scholar]
- 36.Katagiri H., et al. Nonsense mutation of glucokinase gene in late-onset non-insulin-dependent diabetes mellitus. Lancet. 1992;340:1316–1317. doi: 10.1016/0140-6736(92)92494-z. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes C.J. Type 2 diabetes-a matter of β-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 38.Ikemoto S., et al. High fat diet-induced hyperglycemia: prevention by low level expression of a glucose transporter (GLUT4) minigene in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3096–3099. doi: 10.1073/pnas.92.8.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubota N., et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell. . 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 40.Terauchi Y., et al. Increased insulin sensitivity and hypoglycaemia in mice lacking p85α subunit of phosphoinositide 3-kinase. Nat. Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- 41.Terauchi Y., et al. Development of non-insulin-dependent diabetes mellitus in the double knockout mice with disruption of insulin receptor substrate-1 and β-cell glucokinase genes. J. Clin. Invest. . 1997;99:861–866. doi: 10.1172/JCI119250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watada H., et al. The human glucokinase gene β-cell-type promoter: an essential role of insulin promoter factor 1 (IPF1)/PDX-1 in its activation in HIT-T15 cells. Diabetes. . 1996;45:1478–1488. doi: 10.2337/diab.45.11.1478. [DOI] [PubMed] [Google Scholar]
- 43.Kaneto H., et al. Beneficial effects of antioxidants in diabetes. Possible protection of pancreatic β-cells against glucose toxicity. Diabetes. . 1999;48:2398–2406. doi: 10.2337/diabetes.48.12.2398. [DOI] [PubMed] [Google Scholar]
- 44.Ishii C., et al. Beta-cell replication in insulin-induced remission of IDDM in BB/Wor//Tky rats. Diabetes Res. 1996;31:1–18. [Google Scholar]
- 45.Kubota N., et al. Insulin receptor substrate 2 plays a crucial role in β cells and the hypothalamus. J. Clin. Invest. 2004;114:917–927. doi: 10.1172/JCI200421484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eto K., et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 47.Miwa I., et al. Utility of 3-O-methyl-N-acetyl-D-glucosamine, an N-acetylglucosamine kinase inhibotor, for accurate assay of glucokinase in pancreatic islets and liver. . Enzyme Protein. 1995;48:135–142. doi: 10.1159/000474980. [DOI] [PubMed] [Google Scholar]
- 48.Ishii M., et al. Direct comparison of GeneChip and SAGE on the quantitative accuracy in the transcript profiling analysis. Genomics. 2000;68:136–143. doi: 10.1006/geno.2000.6284. [DOI] [PubMed] [Google Scholar]
- 49.Liu W.M., et al. Analysis of high density expression microarrays with signed-rank call algorithms. Bioinformatics. 2002;18:1593–1598. doi: 10.1093/bioinformatics/18.12.1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.