Abstract
Pancreatic stellate cells (PaSCs) are myofibroblast-like cells found in the areas of the pancreas that have exocrine function. PaSCs are regulated by autocrine and paracrine stimuli and share many features with their hepatic counterparts, studies of which have helped further our understanding of PaSC biology. Activation of PaSCs induces them to proliferate, to migrate to sites of tissue damage, to contract and possibly phagocytose, and to synthesize ECM components to promote tissue repair. Sustained activation of PaSCs has an increasingly appreciated role in the fibrosis that is associated with chronic pancreatitis and with pancreatic cancer. Therefore, understanding the biology of PaSCs offers potential therapeutic targets for the treatment and prevention of these diseases.
Introduction
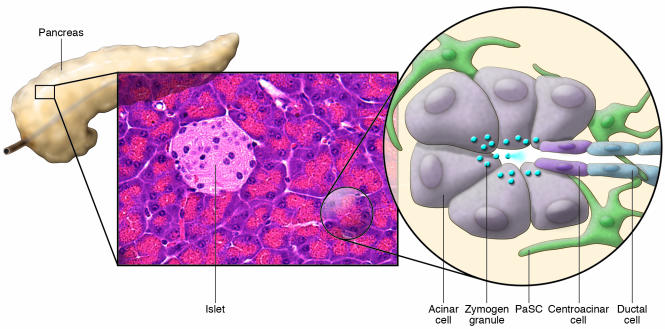
The pancreas can be functionally divided into an exocrine component, which produces enzymes that aid digestion in the gastrointestinal tract, and an endocrine component composed of the islets, which, among other functions, secretes hormones that regulate carbohydrate metabolism. The exocrine component consists primarily of clusters of acinar cells (known as acini) that secrete digestive enzymes into the lumen of the acini. These acini drain the digestive enzymes into the duodenum through the pancreatic ducts (Figure 1). Pancreatic stellate cells (PaSCs) are one of several resident cells in the exocrine pancreas. They are present in the periacinar space and have long cytoplasmic processes that encircle the base of the acinus (Figure 1 and Figure 2, A and B). They can also be found in perivascular and periductal regions of the pancreas (1–4) and serve as key participants in the pathobiology of the major disorders of the exocrine pancreas, including chronic pancreatitis and pancreatic cancer. In these disorders, PaSCs participate in disease pathogenesis after transforming from a quiescent state into an “activated” state (also known as a “myofibroblastic” state).
Figure 1. Schematic of the cellular components of the exocrine pancreas.
The pancreas can be functionally divided into 2 components that are interspersed: an exocrine component that consists primarily of acini — clusters of acinar cells that feed into ductules — and an endocrine component composed of the islets. In the normal pancreas, quiescent PaSCs are present in the periacinar space. These cells have long cytoplasmic processes that encircle the base of the acinus. Zymogen granules release their contents of digestive enzymes into the pancreatic ductal system upon stimulation.
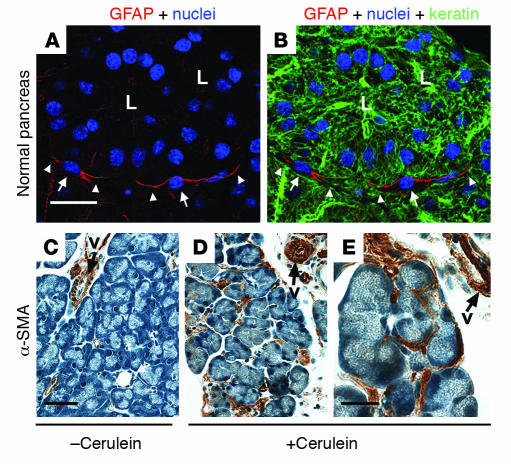
Figure 2. Immune staining of PaSCs.
(A and B) Normal mouse pancreas was triple stained to visualize GFAP (red), nuclei (blue), and keratin polypeptide 8 (green). L, lumen; arrows point to PaSC nuclei and arrowheads point to PaSC processes. (C–E) Pancreata from plasminogen-deficient mice injected with saline (C) or with cerulein to induce pancreatitis (D and E) were stained with antibodies specific for α-SMA as described previously (61). The image shown in E is a higher magnification of that shown in D. Note the dramatic induction of α-SMA in activated PaSCs that surround or are located between acini. Arrows point to blood vessel (v) staining. Scale bars: 20 μm (A and B), 50 μm (C and D), and 20 μm (E).
Approximately 70%–90% of cases of chronic pancreatitis result from alcohol abuse, while the remainder of cases are associated with genetic disorders (for example, hereditary pancreatitis and cystic fibrosis) and unknown causes (for example, idiopathic pancreatitis) (5–8). The course of chronic pancreatitis is characterized by recurrent episodes of acute pancreatitis, which cause parenchymal injury and necrosis, with increasing amounts of fibrosis, chronic inflammation, and parenchymal cell loss with each successive episode. Parenchymal cells in both the exocrine and, to a lesser extent, the endocrine pancreas are lost, and this leads to irreversible and debilitating exocrine, and ultimately endocrine, insufficiency that can be accompanied by a severe chronic pain syndrome. This series of events, which was determined by examination of human pancreatic tissue during alcohol-induced acute and chronic pancreatitis, has been termed the “necrosis-fibrosis sequence” and provides a framework for understanding chronic pancreatitis (9). Adding to the morbidity and mortality of this disorder is the fact that patients with chronic pancreatitis have a substantially increased risk of developing pancreatic cancer (10, 11). Like chronic pancreatitis, adenocarcinoma of the pancreas, which is the most common form of pancreatic cancer, has a remarkable fibrotic component (12–15).
Several excellent reviews have covered the molecular and cellular regulation of pancreatic (16–18) and hepatic (19–22) fibrosis. However, here we consider the important role of the PaSC in the pathogenesis of key disorders of the pancreas. This Review will provide a synopsis of the properties and activation of PaSCs, as well as outline their role in pancreatic inflammation and cancer. We also compare PaSCs with their hepatic counterparts and describe potential PaSC regulatory pathways that might be used to therapeutic advantage.
Properties of PaSCs
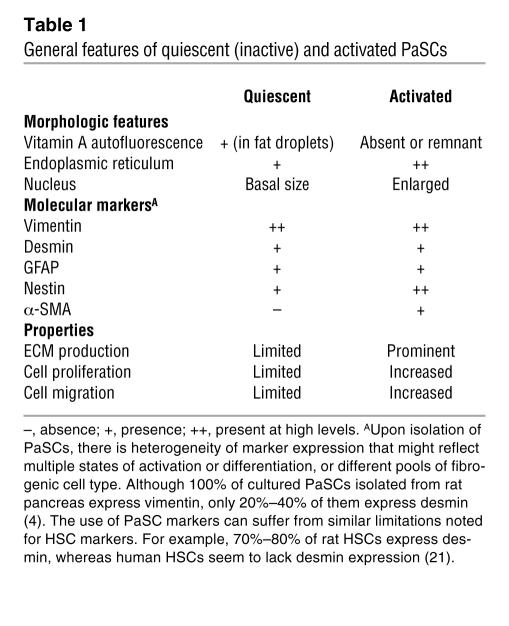
Early studies of PaSCs capitalized on the knowledge and experience gained from study of their hepatic counterparts, the hepatic stellate cells (HSCs), which were first described by Karl von Kupffer in 1876 as Sternzellen (star cells) and initially felt to be phagocytic endothelial cells (reviewed in ref. 21). Stellate cells derive their name from their shape (stella in Latin means “a star”) (Figure 1) and are also present in several other organs, including the kidney (23) and lung (24). Cells in the pancreas that were similar to HSCs in that they were fat-storing cells were first observed with the use of autofluorescence and electron microscopy in 1982 (1). They were identified when rats were given vitamin A, because cells with cytoplasmic fat droplets, such as HSCs (reviewed in ref. 25), become autofluorescent when vitamin A accumulates in these droplets (1). Subsequently, 2 landmark reports described the isolation and initial characterization of what have henceforth been termed PaSCs (3, 4). PaSCs express the intermediate filament proteins desmin and glial fibrillary acidic protein (GFAP) (Figure 2, A and B), which, together with the presence of intracellular fat droplets, serve to distinguish PaSCs from normal fibroblasts (3, 4) (Table 1).
Table 1 .
General features of quiescent (inactive) and activated PaSCs
PaSCs express intermediate filament proteins that usually characterize several cell types — for example, desmin, which characterizes myocytes; GFAP, which characterizes astrocytes; vimentin, which characterizes cells such as leukocytes, fibroblasts, and endothelial cells; and nestin, which characterizes neuroepithelial stem cells (26). The expression of such a diversity of intermediate filament proteins highlights that PaSCs have a broad range of potential properties, including contractility, the presence of cellular extensions to sense their environment, the potential to elaborate ECM components, and the potential to proliferate. However, it is important to note that these markers have clear limitations (see below) and that there are species differences (Table 1). Activation of quiescent PaSCs, which occurs when primary PaSCs are cultured and in the pancreas as a consequence of pancreatic injury, is associated with several morphologic changes (3, 4), including nuclear enlargement and enhanced prominence of the ER network (Table 1). Furthermore, in situ hybridization and immunohistochemical studies indicated that activated PaSCs express α-SMA (also known as ACTA2) (Figure 2, C–E) and collagen type I, therefore marking these cells as a source of fibrosis in chronic pancreatitis and pancreatic adenocarcinoma (14, 27, 28).
Initial efforts to isolate PaSCs produced cells that expressed α-SMA and collagen I, collagen III, and collagen IV (29). However, use of the isolation protocols used in HSC studies, in particular density gradient centrifugation, allowed the isolation of quiescent PaSCs (3, 4). Isolated PaSCs are characterized as quiescent by the presence of desmin, GFAP, and intracellular fat droplets, but the absence of α-SMA (Table 1). Primary PaSCs become activated during culture and attain a myofibroblast-like phenotype characterized by the disappearance of intracellular fat droplets, and the expression of α-SMA and ECM proteins (collagen I, collagen III, and fibronectin) (3, 4) (Table 1). PaSCs also seem to increase their expression of nestin upon activation (30). One potential problem in the identification of PaSCs is that the markers used can also be expressed by other cell types, including PaSC precursors, (myo)fibroblasts, vascular cells, and neural cells. However, on the basis of these markers, PaSCs and HSCs are estimated to constitute nearly 4% and 8% of total pancreatic and hepatic cells, respectively (3, 21).
Although much has been learned from cultures of primary PaSCs, one of the dilemmas in the study of these cells is whether the transformation observed in culture is equivalent to what happens in pancreatic tissue (31). In culture, primary PaSCs continually change from a quiescent to an activated phenotype, and during this change they pass through a series of temporal states of transformation (32). For example, rapidly proliferating PaSCs in culture can either die by apoptosis or acquire a (myo)fibroblastic differentiated state that is more resistant to apoptosis. But how these observations relate to PaSC activation in vivo is unclear, which emphasizes the need for more information about the phenotypic states of PaSCs during disease progression and the mechanisms underlying the conversions between these states.
The propagation of immortalized PaSCs from rat and human pancreata provides additional experimental models to study PaSC biology (33–35) and provides a tool for overexpression and RNA interference studies, as well as a tool for high-throughput screening for compounds that affect PaSC activation. Immortalized cell lines have been generated by expression of either SV40 large T antigen alone in rat PaSCs or SV40 large T antigen and human telomerase in human PaSCs. The resultant immortalized cell lines possess a phenotype consistent with activated PaSCs, which includes expression of α-SMA and ECM proteins. DNA microarrays have been used to compare the gene expression profile of immortalized and primary cultures of rat PaSCs. These revealed only a few overall differences, including differences in the expression of genes encoding ECM-related proteins, cytokines, integrins, and intermediate filament proteins (34). In addition, both rat and human immortalized cell lines responded to TGF-β1, PDGF, and the PPARγ ligand PGJ2 in a manner similar to that of their cultured primary cell counterparts (34–36). The combined use of cultured primary PaSCs and immortalized cells, coupled with the use of coculture systems (for example, coculture of acinar cells and PaSCs), is likely to provide additional mechanistic insights into the biology of PaSCs.
Mediators of PaSC activation
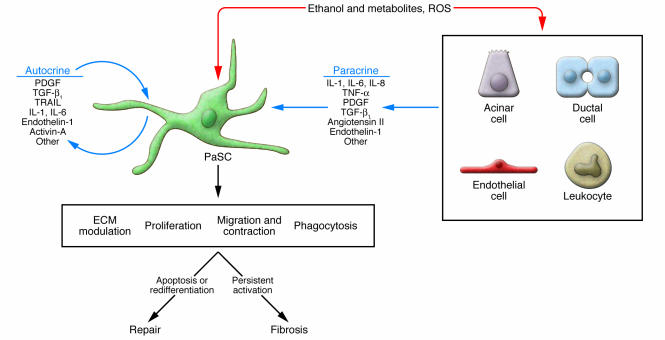
The critical regulatory events that orchestrate the persistent activation of PaSCs in vivo are likely to be similar, at least in part, to the events that regulate the activation of primary PaSCs in culture. Studies of human and rodent primary PaSCs in culture have identified numerous growth factors, cytokines, hormones, intracellular signaling molecules, and transcription factors as regulators of PaSC activation. Potential activators of PaSCs in vivo include paracrine factors, such as cytokines (IL-1, IL-6, IL-8, and TNF-α), growth factors (PDGF and TGF-β1), angiotensin II, and reactive oxygen species released by damaged neighboring cells and leukocytes recruited in response to pancreatic injury (37–43) (Figure 3). Activated PaSCs, in turn, can produce autocrine factors, such as PDGF, TGF-β1, cytokines (IL-1, IL-6, and TRAIL), and proinflammatory molecules (COX-2), that can perpetuate the activated phenotype (40, 42, 44, 45). In addition, activin-A, a member of the TGF-β family of soluble factors, also functions in an autocrine manner, increasing collagen secretion and augmenting TGF-β1 expression and secretion (46). Also, endothelin-1 is expressed by rat PaSCs in primary culture and can stimulate their migration and contraction (47). Although several inflammatory mediators released during pancreatitis have the potential to regulate PaSCs, abundant experimental evidence supports major roles for PDGF (which induces the proliferation of PaSCs and contributes to the migration potential of PaSCs) and TGF-β1 and angiotensin II (which induce PaSCs to express α-SMA and ECM proteins) as modulators of the persistently activated and profibrotic phenotype of these cells (16–18, 37, 41) (Figure 3).
Figure 3. Mechanisms of PaSC activation.
Exposure of the pancreas to ethanol, to its metabolites, and to insults that generate ROS all result in PaSC activation by autocrine and paracrine products. The paracrine factors are derived from neighboring cells such as acinar cells, ductal cells, endothelial cells, and leukocytes. Activated PaSCs can migrate to sites of tissue damage, undergo regulated contraction, proliferate, phagocytose, and generate products that modulate the ECM by facilitating repair or promoting fibrosis (17, 42, 56, 69). Persistent activation of PaSCs promotes fibrosis, while redifferentiation to a quiescent state or stimulation to undergo apoptosis facilitates tissue repair.
Oxidative stress and ethanol metabolites have also been suggested as potential perpetuators of the activated PaSC phenotype. Ethanol can be metabolized in pancreatic acinar cells, resulting in toxic metabolites and oxidative stress that can induce pancreatic damage (48). Cultured rat PaSCs exhibit ethanol-induced alcohol dehydrogenase activity in vitro, suggesting that PaSCs can also metabolize ethanol (48). In vitro studies indicate that ethanol and its metabolite acetaldehyde promote the activation of rat PaSCs and cause lipid peroxidation in these cells (48, 49). Moreover, the antioxidant vitamin E prevents ethanol- and acetaldehyde-induced activation of PaSCs, thereby suggesting that oxidative stress regulates PaSC activation. For example, 4-hydroxy-nonenal, a highly reactive product of lipid peroxidation, activates primary rat PaSCs in culture. These in vitro findings were supported by histological analysis of pancreatic sections from patients with chronic pancreatitis, which showed 4-hydroxy-nonenal staining localized to activated PaSCs within fibrotic areas and to acinar cells adjacent to areas of fibrosis (28).
Multiple studies have identified several major signaling pathways involved in the regulation of PaSC function (27, 43, 47, 50–52). MAPKs are key mediators of activating signals initiated by growth factors, angiotensin II, and ethanol (51, 52). Other signaling pathways regulating PaSC activation include PI3K, RHO kinase, the JAK/STAT pathways, the activator protein-1 and NF-κB pathways, and the TGF-β/SMAD–related pathways (47, 50, 53). In addition, studies using PPARγ ligands have implicated this pathway in the downregulation of PaSC activation (27). These signaling pathways are potential therapeutic targets for the modulation of PaSC function.
PaSCs and pancreatic inflammation
Extensive studies have shown that activated PaSCs in culture express several growth factors and cytokines, as well as the receptors for these molecules, which are known to participate in inflammatory and fibrotic processes. Although the cellular and molecular mechanisms underlying the development of pancreatitis are not completely understood, it is generally accepted that pancreatitis is initiated by damage to acinar, ductal, and/or mesenchymal cells in the pancreas (7, 54, 55). Following damage, pathologic processes that occur in the pancreas include interstitial edema, necrosis of parenchymal cells, intrapancreatic trypsin activation, inflammatory cell infiltration, and activation and proliferation of PaSCs. In human and rodent pancreatic tissues, activated PaSCs are usually found in areas of extensive necrosis and inflammation where an environment rich in cytokines, growth factors, and reactive oxygen species prevails (56–58). Time-course studies using several animal models of experimental pancreatitis (59–61) indicate that parenchymal necrosis and inflammation precede PaSC activation, thereby suggesting that a necrotic, inflammatory process is a prerequisite for activation of these cells. Hence, autocrine and paracrine mediators are probably involved in PaSC activation. In turn, activation facilitates PaSC proliferation, migration, and ECM deposition, which leads to fibrosis or ECM remodeling as part of a repair process (Figure 3).
Evidence of a pivotal role for activated PaSCs in the development of pancreatic fibrosis is based, in part, on the analysis of pancreatic sections from patients with chronic pancreatitis and from experimental pancreatic fibrosis in rodents. For example, α-SMA–expressing cells are abundant in areas of fibrosis in pancreatic tissue sections from patients with chronic pancreatitis of different etiologies (4, 27, 28, 58). In these fibrotic areas, only α-SMA–expressing cells produce mRNA encoding procollagen α1I, indicating that activated PaSCs are probably the predominant source of collagens during pancreatic fibrosis (27). Further evidence for this conclusion comes from the observation that spontaneous chronic pancreatitis in the Wistar Bonn/Kobori (Wbn/Kob) rat is characterized by inflammation and PaSC activation in areas of pancreatic fibrosis (50). In addition, in rats, administration of trinitrobenzene sulfonic acid into the pancreatic duct, as well as i.v. injection of dibutyltin dichloride, induces necrosis and inflammation of the pancreas followed by PaSC activation and fibrosis (27, 62). Similarly, mouse pancreatitis induced with the use of cerulein (a cholecystokinin analogue and pancreas secretagogue) is also accompanied by PaSC activation and fibrosis (63, 64).
The involvement of PaSCs in the regulation of the inflammatory response during pancreatitis occurs, at least in part, through their production of chemokines and cytokines, as has been reported for myofibroblast-type cells in other tissues (65–68). PaSCs have also been shown to have phagocytic activity in vivo and in cell culture (69) and therefore might also function as resident phagocytic cells during pancreatitis. PaSC phagocytic activity is regulated by PPARγ and expression of CD36, a scavenger receptor that promotes phagocytosis. Interestingly, profibrogenic cytokines, such as TGF-β, TNF-α, and IL-1, decreased the phagocytic capacity of PaSCs activated in culture (69). These results highlight the complex regulation of the profibrotic and proinflammatory properties of PaSCs during pancreatic inflammation.
Inflammation and PaSC modulation of the ECM
PaSCs have a key role in the extensive tissue fibrosis that accompanies chronic pancreatitis and leads to destruction of the pancreas and loss of exocrine function (17, 56, 58). Conversely, observational studies have shown that activated PaSCs participate in tissue repair processes after acute necrotizing pancreatitis in humans and experimental acute pancreatitis in rodents (59–61, 70). Although the exact role of PaSCs in pancreatic repair and regeneration remains to be elucidated, activated PaSCs and other myofibroblast cells resident in the pancreas probably contribute to the formation of a provisional matrix at the site of injury that allows cell proliferation, migration, and assembly of new parenchymal cells (60, 61, 71). In addition, PaSCs can regulate ECM remodeling during pancreatic tissue repair through the production of ECM-degrading proteases and their inhibitors, such as tissue inhibitor of metalloproteinase 1 (TIMP-1) (40, 72). In this context, resolution of mouse cerulein-induced pancreatitis involves transient activation of PaSCs and deposition of ECM proteins, as well as transient upregulation of MMPs and TIMP-1 (61). Similar regulation of ECM remodeling by HSCs is also likely to mediate liver regeneration after experimental injury (20, 73).
In most studies in which PaSCs are activated after damage to the pancreas, the inflammatory process resolves and activated PaSCs progressively disappear after the cessation of the injurious agent. However, repeated pancreatic damage and failure of the mechanisms regulating tissue repair can both lead to chronic inflammation, persistent activation, proliferation of PaSCs, and finally fibrosis. Consistent with this hypothesis, repeated episodes of acute experimental pancreatitis produce changes that resemble those found in chronic pancreatitis (63, 74). In fact, fibrosis in the pancreas and other organs can be considered the consequence of a wound-healing process responding to chronic injurious stimuli. In humans, repeated damage to the pancreas is associated with chronic alcohol consumption, pancreatic duct obstruction, metabolic disorders, and genetic defects (7, 75). The chronic injury results in perpetuation of the activated PaSC phenotype. Furthermore, chronic pancreatitis is associated with reduced production of MMPs by PaSCs, which probably helps promote and sustain the fibrotic phenotype (40).
In addition to the multiple factors described above, other mechanisms can explain the persistent activated state of PaSCs during pancreatitis. For example, activated PaSCs express protease-activated receptor-2 (PAR-2), which is cleaved by trypsin (a key pathogenic protease in pancreatitis) to become active. Active PAR-2 stimulates PaSC proliferation and collagen synthesis (76). Changes in the composition of the ECM during repair processes can also modulate PaSC activation. For example, activated PaSCs revert to a quiescent state when cultured on a basal membrane–like matrix, thereby suggesting that ECM composition regulates PaSC behavior (35, 40). Studies of liver fibrosis have shown that extensive ECM degradation is accompanied by apoptosis of HSCs, as a result of either increased proapoptotic signaling or reduced survival signals from the ECM (77), but it remains to be shown whether this is also true in the pancreas. More recently, impaired extracellular proteolysis of the ECM in mice lacking plasminogen has been associated with persistent PaSC activation and accumulation of pancreatic collagens during the recovery phase of mouse cerulein-induced pancreatitis (61). Moreover, levels of pancreatic TGF-β1, plasminogen activator inhibitor-1, and TIMP-1, factors that promote fibrosis (37, 77), were elevated persistently in plasminogen-deficient mice after treatment with cerulein but only transiently in similarly treated plasminogen-sufficient mice (61).
PaSCs and pancreatic cancer
Ductal pancreatic adenocarcinoma, which is the most common form of pancreatic cancer, is the fourth leading cause of cancer-related death, with a 5-year survival of less than 5% (78). Several tumors, and in particular pancreatic adenocarcinomas, are characterized by “tumor desmoplasia,” a remarkable increase in connective tissue that infiltrates and envelopes the neoplasm (79). Activated PaSCs in the tumor desmoplasia of human pancreatic cancers express α-SMA and colocalize with mRNA encoding procollagen α1I (14) and are probably major contributors of the ECM proteins that constitute the desmoplasia (12, 14, 80–84). Of note, analysis of gene expression in human pancreatic adenocarcinoma, chronic pancreatitis, normal pancreas, and pancreatic cancer cell lines demonstrated that 107 genes predicted to be expressed in the stromal compartment were found in both pancreatic adenocarcinoma and chronic pancreatitis (81). Furthermore, isolation and characterization of stromal cells from human pancreatic adenocarcinoma and alcohol-induced chronic pancreatitis samples demonstrated that cells from both sources had the same characteristic morphology, cytofilament expression, and capacity to synthesize ECM proteins (84). These results demonstrate that these 2 disorders contain common stromal elements and suggest similar mechanisms underlying the development of fibrosis in chronic pancreatitis and the desmoplasia in pancreatic adenocarcinoma.
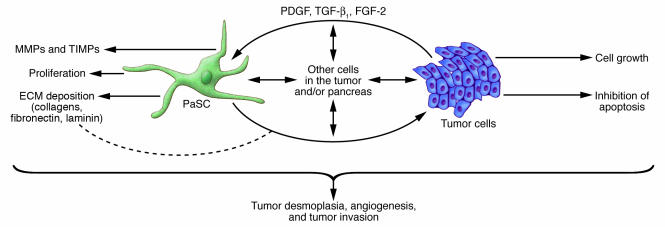
Evidence is emerging that there is a symbiotic relationship between pancreatic adenocarcinoma cells and PaSCs that results in an overall increase in the rate of growth of the tumor (Figure 4). For example, culture supernatants from human pancreatic tumor cell lines stimulate PaSC proliferation and production of ECM proteins (14, 82–84). In addition, the growth rate of tumor cells injected s.c. into nude mice (mice that lack T cells and are severely immunocompromised) is markedly increased when PaSCs are included in the inoculum (84). In contrast to the tumors that form when only cancer cells are injected, the tumors that form when both cancer cells and PaSCs are used have a desmoplasia similar to that observed in human pancreatic adenocarcinoma (84). Furthermore, pancreatic tumor cells induce the proliferation of PaSCs by secreting PDGF and induce PaSC production of ECM proteins by secreting TGF-β1 and FGF-2 (84).
Figure 4. Effect of PaSCs on tumor cell invasion and desmoplasia.
There is accumulating evidence for PaSC cross-talk with tumor cells, which in turn contributes to the profound tumor desmoplasia that is noted in pancreatic cancer (12, 14, 80–84). Such direct or indirect cell-cell dialogue can also promote tumor invasion and possibly angiogenesis, although a role in angiogenesis has been more studied in the liver. Other cells in the pancreas (for example acinar cells, ductal cells, endothelial cells, and leukocytes) are likely to be involved.
Although studies using animals indicate that pancreatic tumor cells and PaSCs promote each other’s proliferation, supporting data from human pancreatic tumors are limited and inconsistent. One report shows that more extensive intratumoral fibroblastic cell proliferation correlates with a poorer disease outcome (85). Another report shows that patients with a better outcome have increased expression of connective tissue growth factor (CTGF) in fibroblasts surrounding the human pancreatic tumor cells (86). CTGF has been implicated in the pathogenesis of fibrotic diseases, and its expression is predominant in PaSCs and is regulated by TGF-β. These early findings are intriguing and suggest that further work is needed to delineate the role of PaSCs and their regulators in pancreatic cancer.
The mechanisms by which PaSCs and the desmoplasia enhance the growth of tumor cells in the adenocarcinomas are complex and only partly understood. One role of the desmoplasia is to promote survival and prevent apoptosis of the tumor cells through a direct action of ECM proteins on the tumor cells (87, 88) (Figure 4). The prosurvival effects of the ECM proteins laminin and fibronectin are mediated through their integrin receptors, which are expressed by the tumor cells. In addition, the effects of fibronectin seem to be mediated through transactivation of IGF-1. Overall, these interactions lead to the activation of prosurvival and progrowth signaling pathways in pancreatic tumor cells (88, 89).
Another possible mechanism by which tumor desmoplasia might promote pancreatic adenocarcinoma cell growth is that PaSCs and tumor cells produce MMPs and tissue serine proteases (Figure 4), such as members of the plasminogen activator system, that degrade ECM proteins (61, 72, 90). In this context, MMPs and tissue serine proteases might promote tumor cell invasion and metastasis, as has been postulated in other cancers (90–93).
Comparison of PaSCs and HSCs
Although PaSCs and HSCs share several morphologic and marker features, some differences exist. Unique, context-related features of HSCs include the portal perfusion and generally abundant vascular flow in the liver, and their proximity to encounter endothelial cell cross-talk due to their hepatic location within the subendothelial space (19). In addition, a genome-wide assessment of gene expression that included 21,329 genes identified 29 that were differentially expressed between cultures of primary HSCs and PaSCs (94). However, the fact that the overwhelming majority of genes showed no difference in expression supports the notion that these 2 cell types are very similar. Recent studies have revealed that the bone marrow is the source of 68% of HSCs and 70% of myofibroblast cells in mouse models of carbon tetrachloride– and thioacetamide-induced fibrosis (95). Whether the same holds true in the pancreas remains to be determined, but a common origin in the bone marrow could account for the strong similarities between HSCs and PaSCs.
Although this Review has concentrated on stellate cells, other potential sources of fibrosis are present in both the pancreas and the liver. For example, hepatic myofibroblasts, which are distinguished from HSCs by their location within the liver and their expression of the ECM component fibulin-2 but not GFAP (96, 97), and tissue fibroblasts, which express vimentin but not GFAP and desmin, represent an additional source of ECM proteins (96–100). Subpopulations of fibroblastic cells, which might reflect distinct levels of fibroblast activation, can be distinguished in cirrhotic livers on the basis of differing levels of cellular retinol-binding protein-1 and α-SMA (101), and these cells might also produce ECM proteins. The origin of these liver myofibroblast-type cells and similar cells in the pancreas remains to be conclusively determined. Bone marrow transplantation in a mouse model of pancreatic insulinoma demonstrated that the bone marrow is the source of 25% of pancreatic myofibroblasts and is likely to contribute to tumor-associated fibrosis (102). In addition, nestin-positive and nestin-negative precursors isolated from adult mouse pancreas are able to differentiate in vitro to yield multiple lineages of the pancreas, including PaSCs (103). The relative contributions of bone marrow and resident precursors to the PaSC population, as well as to the other myofibroblast- and fibroblast-related cell populations, remain to be determined.
The function of quiescent PaSCs is still being investigated, but clues can be gleaned from studies involving HSCs (20–22). For example, HSCs have been implicated in several important liver physiologic roles, under basal conditions, that include vitamin A storage; production and turnover of normal ECM proteins; and communication with hepatocytes via gap junctions and the production of paracrine factors to promote hepatocyte differentiation, and to regulate ductal and vascular pressures. Similar roles can be envisioned for PaSCs in the inactive state. For example, perivascular and periductal PaSCs (2) might regulate the pressure within these compartments by contraction mediated by endothelin-1 (47). The functions of PaSCs, by analogy to HSCs, should be envisioned not only in isolation but also in the context and under the influence of their microenvironment.
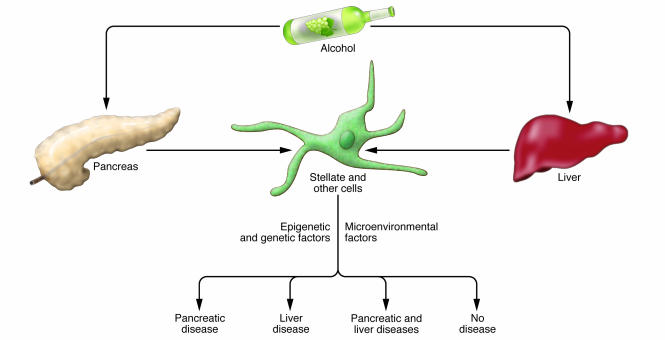
Although alcohol and/or its metabolites have been shown to similarly activate stellate cells of the pancreas and liver, it is unclear why substantial differences exist in an individual’s susceptibility to liver and pancreas fibrosis (Figure 5). Potential differences between the pancreas and the liver after injury, in addition to the apparent difference in the cellular microenvironment, include edema formation during pancreatitis; different ethanol metabolism in acinar cells and hepatocytes; and different lipid metabolism between acinar cells and hepatocytes (for example, “fatty” pancreas has not been described). Given that the stellate cell is an integral element of the onset of these diseases, microenvironment differences and other genetic and epigenetic modifiers related to stellate and/or other resident and infiltrating cells are likely to be important (Figure 5), but this hypothesis remains to be tested. For example, comparison of the response of PaSCs and HSCs isolated from the same animal with parallel comparative responses in different genetic backgrounds has not been reported. Furthermore, attempts at assessing genetic polymorphisms (for example, in enzymes such as cytochrome P450 2E1 and aldehyde dehydrogenase-2) that may promote susceptibility to alcoholic chronic pancreatitis or cirrhosis have not been overly revealing (104). However, an understanding of genetic polymorphisms that predispose to chronic pancreatitis (8, 75) or liver fibrosis (105, 106), or even protect from chronic pancreatitis (107), is being pursued. A systematic and broad-based genetic screening will be needed, using well-defined animal models and patient cohorts. Proteomic comparisons between PaSCs and HSCs, which are likely to be forthcoming, should also provide additional insights.
Figure 5. Differential impact of alcohol on the pancreas and liver.
The effects of alcohol and its metabolites on the pancreas and liver can vary substantially from one individual to another, with some showing no effects, some having a pancreas-selective or liver-selective effect, and yet others having disease in both organs. Poorly understood genetic, epigenetic, and microenvironmental factors are likely to be responsible for these dramatic differences.
Potential treatments for PaSC disorders
In concept, treatments for disorders involving PaSCs (that is, chronic pancreatitis and pancreatic cancer) should target the key mechanisms involved in their activation and proliferation. For example, blockade of the receptors for PDGF, TGF-β, and angiotensin II, as well as blockade of the intracellular signaling pathways downstream of these receptors, is likely to provide therapeutic benefit (17, 51, 76, 108). In this regard, there are in vivo experiments showing the importance of the angiotensin II system in the development of pancreatic fibrosis (64, 108). There are also several reports from in vitro experiments using PaSCs that show key roles in the activation and/or proliferation process for MAPK pathways, in particular, ERK1/2, p38 kinase, and JNK (50, 52, 53, 66, 109, 110); PI3K and PKC (111); PPARγ (36, 112); NADPH oxidases (our unpublished observations); and ethanol metabolism to acetaldehyde (18). In these studies, inhibition of most of these pathways results in attenuation of the activation and proliferation of PaSCs, but activation of PPARγ seems to block PaSC activation (36, 112). It has also been reported that activated rat PaSCs express COX-2 when stimulated with TGF-β1 and other cytokines (45), as well as when stimulated with conditioned media from human pancreatic tumor cells (113). Pharmacological inhibition of COX-2 and blockade of the TGF-β1 signaling pathway decrease the expression of COX-2, α-SMA, and collagen I, suggesting that COX-2 might be a relevant therapeutic target for chronic pancreatitis and pancreatic cancer. Furthermore, efforts aimed at inducing PaSC transdifferentiation from an activated to a quiescent state and at inducing PaSC apoptosis are also attractive modalities. For example, administration of vitamin A (retinol and its metabolites) induces culture-activated rat PaSCs to become quiescent (114).
In vivo studies using animal models of chronic pancreatitis have demonstrated that several agents have beneficial effects (115), but few in vivo studies have assessed whether targeting PaSCs in the desmoplastic reaction of pancreatic cancer has beneficial effects. In the case of targeting fibrosis in pancreatic tumor models, antibodies to CTGF, which activate PaSCs through their α5β1 integrin receptor (44), inhibited tumor growth and metastasis in a xenograft mouse pancreatic tumor model (116, 117). Agents showing benefit in experimental chronic pancreatitis include inhibitors of angiotensin II–converting enzyme and antagonists of the angiotensin II receptor (50, 108); vitamin E (118) and DA-9601 (119), which function as antioxidants; troglitazone and thiazolidinedione, which are PPARγ ligands (120, 121); camostat, which is a serine protease inhibitor (62, 122); the herbal preparation Saiko-keishi-to, which has antioxidant and antiinflammatory properties (123); and COX-2 inhibitors (124). In addition, strategies aimed at blocking TGF-β signaling pathways (113, 125) have proved effective in reducing experimental pancreatic fibrosis in rodents. Considering the importance of PaSC pathobiology in both chronic pancreatitis and pancreatic cancer, there should be increased effort devoted to addressing PaSC functions in these disorders and the development of therapeutic strategies that include targeting the PaSC.
Concluding remarks
The PaSC is a recently identified cell in the pancreas, which explains why a PubMed search for “pancreatic stellate cells” provides 177 total citations, with a substantially rising trajectory (for example, 64 citations in 2004 and 2005 versus 45 citations in 2003 and 2004), as compared with 1,806 total citations for “hepatic stellate cells.” Presently, much remains to be learned about the biology of PaSCs and their role in diseases of the pancreas. Indeed, we need a better understanding of their function during the quiescent state, their regulation in terms of activation and deactivation (that is, differentiation and transdifferentiation, respectively), their elimination (for example, through apoptosis), their cross-talk with neighboring cells, and their origin. Such understanding will promote and refine therapeutic approaches targeting PaSCs for disorders such as pancreatitis and pancreatic cancers.
Acknowledgments
We are very grateful to Diana Toivola for preparing the immunofluorescence figure and for input during manuscript preparation; Yoon Jung and Carolyn Taylor for assistance with the references; Kris Morrow for preparing the figures; and Minote Apte, Scott Friedman, Roland Schmid, and Hide Tsukamoto for essential comments on the manuscript. Our work is supported by NIH grants DK52951, DK47918, and DK73909 and a VA Merit Award (to M.B. Omary); Stanford University NIH Digestive Disease Center grant DK56339; NIH grant AA15781 (to A. Lugea); the Susan E. Riley and Edna E. Riley Charitable Foundation (to A.W. Lowe); and USC-UCLA NIH Research Center for Alcoholic Liver and Pancreatic Diseases award AA11999. We apologize for being unable to include all the references related to this field because of space limitation.
Footnotes
Nonstandard abbreviations used: CTGF, connective tissue growth factor; GFAP, glial fibrillary acidic protein; HSC, hepatic stellate cell; PaSC, pancreatic stellate cell; TIMP-1, tissue inhibitor of metalloproteinase 1.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:50–59 (2007). doi:10.1172/JCI30082.
References
- 1.Watari N., Hotta Y., Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat. Jpn. 1982;58:837–858. doi: 10.2535/ofaj1936.58.4-6_837. [DOI] [PubMed] [Google Scholar]
- 2.Ikejiri N. The vitamin A-storing cells in the human and rat pancreas. Kurume Med. J. 1990;37:67–81. doi: 10.2739/kurumemedj.37.67. [DOI] [PubMed] [Google Scholar]
- 3.Apte M.V., et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Cancer Res. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachem M.G., et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 5.Whitcomb D.C. Inflammation and cancer. V. Chronic pancreatitis and pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 6.Draganov P., Forsmark C.E. “Idiopathic” pancreatitis. Gastroenterology. 2005;128:756–763. doi: 10.1053/j.gastro.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Dimagno M.J., Dimagno E.P. Chronic pancreatitis. Curr. Opin. Gastroenterol. 2006;22:487–497. doi: 10.1097/01.mog.0000239862.96833.89. [DOI] [PubMed] [Google Scholar]
- 8.Weiss F.U., Simon P., Mayerle J., Kraft M., Lerch M.M. Germline mutations and gene polymorphism associated with human pancreatitis. Endocrinol. Metab. Clin. North Am. 2006;35:289–302, viii–ix. doi: 10.1016/j.ecl.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Comfort M.W., Gambill E.E., Baggenstoss A.H. Chronic relapsing pancreatitis: a study of 29 cases without associated disease of the biliary or gastro-intestinal tract. Gastroenterology. 1946;6:239–285. [PubMed] [Google Scholar]
- 10.Lowenfels A.B., et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 11.Go V.L., Gukovskaya A., Pandol S.J. Alcohol and pancreatic cancer. Alcohol. 2005;35:205–211. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Yen T.W., et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–134. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- 13.Logsdon C.D., et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 14.Apte M.V., et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Schneider G., Siveke J.T., Eckel F., Schmid R.M. Pancreatic cancer: basic and clinical aspects. Gastroenterology. 2005;128:1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Menke A., Adler G. TGFbeta-induced fibrogenesis of the pancreas. Int. J. Gastrointest. Cancer. 2002;31:41–46. doi: 10.1385/IJGC:31:1-3:41. [DOI] [PubMed] [Google Scholar]
- 17.Jaster R. Molecular regulation of pancreatic stellate cell function. Mol. Cancer. 2004;3:26. doi: 10.1186/1476-4598-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apte M.V., Wilson J.S. Mechanisms of pancreatic fibrosis. Dig. Dis. 2004;22:273–279. doi: 10.1159/000082799. [DOI] [PubMed] [Google Scholar]
- 19.Friedman S.L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 20.Bissell D.M. Hepatic fibrosis as wound repair: a progress report. J. Gastroenterol. 1998;33:295–302. doi: 10.1007/s005350050087. [DOI] [PubMed] [Google Scholar]
- 21.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 22.Tsukamoto H. Adipogenic phenotype of hepatic stellate cells. Alcohol. Clin. Exp. Res. 2005;29(Suppl. 11):132S–133S. doi: 10.1097/01.alc.0000189279.92602.f0. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 24.Keane M.P., Strieter R.M., Belperio J.A. Mechanisms and mediators of pulmonary fibrosis. Crit. Rev. Immunol. 2005;25:429–463. doi: 10.1615/critrevimmunol.v25.i6.10. [DOI] [PubMed] [Google Scholar]
- 25.Wake K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int. Rev. Cytol. 1980;66:303–353. doi: 10.1016/s0074-7696(08)61977-4. [DOI] [PubMed] [Google Scholar]
- 26.Omary M.B., Coulombe P.A., McLean W.H. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 27.Haber P.S., et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casini A., et al. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J. Pathol. 2000;192:81–89. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH675>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Saotome T., Inoue H., Fujimiya M., Fujiyama Y., Bamba T. Morphological and immunocytochemical identification of periacinar fibroblast-like cells derived from human pancreatic acini. Pancreas. 1997;14:373–382. doi: 10.1097/00006676-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Lardon J., Rooman I., Bouwens L. Nestin expression in pancreatic stellate cells and angiogenic endothelial cells. Histochem. Cell Biol. 2002;117:535–540. doi: 10.1007/s00418-002-0412-4. [DOI] [PubMed] [Google Scholar]
- 31.Pandol S.J. Are we studying the correct state of the stellate cell to elucidate mechanisms of chronic pancreatitis? Gut. 2005;54:744–745. doi: 10.1136/gut.2004.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manapov F., Muller P., Rychly J. Translocation of p21(Cip1/WAF1) from the nucleus to the cytoplasm correlates with pancreatic myofibroblast to fibroblast cell conversion. Gut. 2005;54:814–822. doi: 10.1136/gut.2003.036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masamune A., Satoh M., Kikuta K., Suzuki N., Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J. Gastroenterol. 2003;9:2751–2758. doi: 10.3748/wjg.v9.i12.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparmann G., et al. Generation and characterization of immortalized rat pancreatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G211–G219. doi: 10.1152/ajpgi.00347.2003. [DOI] [PubMed] [Google Scholar]
- 35.Jesnowski R., et al. Immortalization of pancreatic stellate cells as an in vitro model of pancreatic fibrosis: deactivation is induced by matrigel and N-acetylcysteine. Lab. Invest. 2005;85:1276–1291. doi: 10.1038/labinvest.3700329. [DOI] [PubMed] [Google Scholar]
- 36.Jaster R., et al. Peroxisome proliferator-activated receptor gamma overexpression inhibits pro-fibrogenic activities of immortalised rat pancreatic stellate cells. J. Cell. Mol. Med. 2005;9:670–682. doi: 10.1111/j.1582-4934.2005.tb00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apte M.V., et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534–541. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luttenberger T., et al. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab. Invest. 2000;80:47–55. doi: 10.1038/labinvest.3780007. [DOI] [PubMed] [Google Scholar]
- 39.Schneider E., et al. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am. J. Physiol. Cell Physiol. 2001;281:C532–C543. doi: 10.1152/ajpcell.2001.281.2.C532. [DOI] [PubMed] [Google Scholar]
- 40.Shek F.W., et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am. J. Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mews P., et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips P.A., et al. Cell migration: a novel aspect of pancreatic stellate cell biology. Gut. 2003;52:677–682. doi: 10.1136/gut.52.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hama K., et al. Angiotensin II promotes the proliferation of activated pancreatic stellate cells by Smad7 induction through a protein kinase C pathway. Biochem. Biophys. Res. Commun. 2006;340:742–750. doi: 10.1016/j.bbrc.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 44.Gao R., Brigstock D.R. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin alpha5beta1 as a novel CCN2 receptor. Gastroenterology. 2005;129:1019–1030. doi: 10.1053/j.gastro.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 45.Aoki H., et al. Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to pro-inflammatory cytokines. Am. J. Physiol. Cell Physiol. 2007 doi: 10.1152/ajpcell.00030.2006. In press. [DOI] [PubMed] [Google Scholar]
- 46.Ohnishi N., et al. Activin A is an autocrine activator of rat pancreatic stellate cells: potential therapeutic role of follistatin for pancreatic fibrosis. Gut. 2003;52:1487–1493. doi: 10.1136/gut.52.10.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masamune A., Satoh M., Kikuta K., Suzuki N., Shimosegawa T. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J. Gastroenterol. 2005;11:6144–6151. doi: 10.3748/wjg.v11.i39.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Apte M.V., Pirola R.C., Wilson J.S. Battle-scarred pancreas: role of alcohol and pancreatic stellate cells in pancreatic fibrosis. J. Gastroenterol. Hepatol. 2006;21(Suppl. 3):S97–S101. doi: 10.1111/j.1440-1746.2006.04587.x. [DOI] [PubMed] [Google Scholar]
- 49. Pandol, S., et al. 2006. Alcohol, reactive oxygen species, pancreatitis and pancreatic cancer. In Alcohol, tobacco, and cancer. C.H. Cho and V. Purohit, editors. Karger. Basel, Switzerland. 109–118. [Google Scholar]
- 50.Kuno A., et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male Wistar Bonn/Kobori rats. Gastroenterology. 2003;124:1010–1019. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- 51.Ohnishi H., et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J. Biol. Chem. 2004;279:8873–8878. doi: 10.1074/jbc.M309698200. [DOI] [PubMed] [Google Scholar]
- 52.Kikuta K., et al. Hydrogen peroxide activates activator protein-1 and mitogen-activated protein kinases in pancreatic stellate cells. Mol. Cell. Biochem. 2006;29:111–120. doi: 10.1007/s11010-006-9189-4. [DOI] [PubMed] [Google Scholar]
- 53.Masamune A., et al. A c-Jun NH2-terminal kinase inhibitor SP600125 (anthra[1,9-cd]pyrazole-6 (2H)-one) blocks activation of pancreatic stellate cells. J. Pharmacol. Exp. Ther. 2004;310:520–527. doi: 10.1124/jpet.104.067280. [DOI] [PubMed] [Google Scholar]
- 54.Stevens T., Conwell D.L., Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am. J. Gastroenterol. 2004;99:2256–2270. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 55.Ruthenburger M., Mayerle J., Lerch M.M. Cell biology of pancreatic proteases. Endocrinol. Metab. Clin. North Am. 2006;35:313–331, ix. doi: 10.1016/j.ecl.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Apte M.V., Wilson J.S. Stellate cell activation in alcoholic pancreatitis. Pancreas. 2003;27:316–320. doi: 10.1097/00006676-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Bentrem D.J., Joehl R.J. Pancreas: healing response in critical illness. Crit. Care Med. 2003;31(Suppl. 8):S582–S589. doi: 10.1097/01.CCM.0000081428.35729.73. [DOI] [PubMed] [Google Scholar]
- 58.Kloppel G., Detlefsen S., Feyerabend B. Fibrosis of the pancreas: the initial tissue damage and the resulting pattern. Virchows Arch. 2004;445:1–8. doi: 10.1007/s00428-004-1021-5. [DOI] [PubMed] [Google Scholar]
- 59.Yokota T., et al. Pancreatic stellate cell activation and MMP production in experimental pancreatic fibrosis. J. Surg. Res. 2002;104:106–111. doi: 10.1006/jsre.2002.6403. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann A., et al. Pancreatic stellate cells contribute to regeneration early after acute necrotising pancreatitis in humans. Gut. 2002;51:574–578. doi: 10.1136/gut.51.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lugea A., et al. Pancreas recovery following cerulein-induced pancreatitis is impaired in plasminogen-deficient mice. Gastroenterology. 2006;131:885–899. doi: 10.1053/j.gastro.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibo J., et al. Camostat mesilate attenuates pancreatic fibrosis via inhibition of monocytes and pancreatic stellate cells activity. Lab. Invest. 2005;85:75–89. doi: 10.1038/labinvest.3700203. [DOI] [PubMed] [Google Scholar]
- 63.Neuschwander-Tetri B.A., Bridle K.R., Wells L.D., Marcu M., Ramm G.A. Repetitive acute pancreatic injury in the mouse induces procollagen alpha1(I) expression colocalized to pancreatic stellate cells. Lab. Invest. 2000;80:143–150. doi: 10.1038/labinvest.3780018. [DOI] [PubMed] [Google Scholar]
- 64.Nagashio Y., et al. Angiotensin II type 1 receptor interaction is an important regulator for the development of pancreatic fibrosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G170–G177. doi: 10.1152/ajpgi.00005.2004. [DOI] [PubMed] [Google Scholar]
- 65.Andoh A., et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology. 2000;119:211–219. doi: 10.1053/gast.2000.8538. [DOI] [PubMed] [Google Scholar]
- 66.Masamune A., Kikuta K., Satoh M., Satoh A., Shimosegawa T. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J. Pharmacol. Exp. Ther. 2002;302:36–42. doi: 10.1124/jpet.302.1.36. [DOI] [PubMed] [Google Scholar]
- 67.Sparmann G., et al. Inhibition of lymphocyte apoptosis by pancreatic stellate cells: impact of interleukin-15. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G842–G851. doi: 10.1152/ajpgi.00483.2004. [DOI] [PubMed] [Google Scholar]
- 68.Aoki H., et al. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am. J. Physiol. Cell Physiol. 2006;290:C1100–C1108. doi: 10.1152/ajpcell.00465.2005. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu K., Kobayashi M., Tahara J., Shiratori K. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128:2105–2118. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 70.Kishi S., et al. Pancreatic duct obstruction itself induces expression of alpha smooth muscle actin in pancreatic stellate cells. J. Surg. Res. 2003;114:6–14. doi: 10.1016/s0022-4804(03)00153-7. [DOI] [PubMed] [Google Scholar]
- 71.Demols A., et al. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G1105–G1112. doi: 10.1152/ajpgi.00431.2001. [DOI] [PubMed] [Google Scholar]
- 72.Phillips P.A., et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut. 2003;52:275–282. doi: 10.1136/gut.52.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedman S.L., Bansal M.B. Reversal of hepatic fibrosis: fact or fantasy? Hepatology. 2006;43(Suppl. 1):S82–S88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 74.Perides G., Tao X., West N., Sharma A., Steer M.L. A mouse model of ethanol dependent pancreatic fibrosis. Gut. 2005;54:1461–1467. doi: 10.1136/gut.2004.062919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitcomb D.C. Mechanisms of disease: advances in understanding the mechanisms leading to chronic pancreatitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004;1:46–52. doi: 10.1038/ncpgasthep0025. [DOI] [PubMed] [Google Scholar]
- 76.Masamune A., Kikuta K., Satoh M., Suzuki N., Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J. Pharmacol. Exp. Ther. 2005;312:651–658. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 77.Issa R., et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Jemal A., et al. Cancer statistics, 2006. CA Cancer J. Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 79.Mollenhauer J., Roether I., Kern H.F. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong T., et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 81.Binkley C.E., et al. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Koninger J., et al. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem. Biophys. Res. Commun. 2004;322:943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida S., et al. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem. Biophys. Res. Commun. 2004;323:1241–1245. doi: 10.1016/j.bbrc.2004.08.229. [DOI] [PubMed] [Google Scholar]
- 84.Bachem M.G., et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe I., et al. Advanced pancreatic ductal cancer: fibrotic focus and beta-catenin expression correlate with outcome. Pancreas. 2003;26:326–333. doi: 10.1097/00006676-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 86.Hartel M., et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J. Surg. 2004;28:818–825. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 87.Vaquero E.C., et al. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188–1202. doi: 10.1016/s0016-5085(03)01203-4. [DOI] [PubMed] [Google Scholar]
- 88.Edderkaoui M., et al. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G1137–G1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 89.Vaquero E.C., Edderkaoui M., Pandol S.J., Gukovsky I., Gukovskaya A.S. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J. Biol. Chem. 2004;279:34643–34654. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 90.Munshi H.G., Stack M.S. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto H., et al. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290–1296. doi: 10.1016/s0016-5085(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 92.Sternlicht M.D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liotta L.A., Kohn E.C. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 94.Buchholz M., et al. Transcriptome analysis of human hepatic and pancreatic stellate cells: organ-specific variations of a common transcriptional phenotype. J. Mol. Med. 2005;83:795–805. doi: 10.1007/s00109-005-0680-2. [DOI] [PubMed] [Google Scholar]
- 95.Russo F.P., et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 96.Knittel T., et al. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–1221. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 97.Knittel T., et al. Localization of liver myofibroblasts and hepatic stellate cells in normal and diseased rat livers: distinct roles of (myo-)fibroblast subpopulations in hepatic tissue repair. Histochem. Cell Biol. 1999;112:387–401. doi: 10.1007/s004180050421. [DOI] [PubMed] [Google Scholar]
- 98.Uchio K., et al. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab. Invest. 2002;82:619–628. doi: 10.1038/labinvest.3780456. [DOI] [PubMed] [Google Scholar]
- 99.Baba S., et al. Commitment of bone marrow cells to hepatic stellate cells in mouse. J. Hepatol. 2004;40:255–260. doi: 10.1016/j.jhep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 100.Forbes S.J., et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 101.Lepreux S., et al. Cellular retinol-binding protein-1 expression in normal and fibrotic/cirrhotic human liver: different patterns of expression in hepatic stellate cells and (myo)fibroblast subpopulations. J. Hepatol. 2004;40:774–780. doi: 10.1016/j.jhep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Direkze N.C., et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 103.Seaberg R.M., et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat. Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 104.Horie Y., Ishii H. What factors play a crucial role in the pathogenesis of alcohol-related chronic pancreatitis and liver cirrhosis? J. Gastroenterol. 2004;39:915–917. doi: 10.1007/s00535-004-1420-z. [DOI] [PubMed] [Google Scholar]
- 105.Huang H., et al. Identification of two gene variants associated with risk of advanced fibrosis in patients with chronic hepatitis C. Gastroenterology. 2006;130:1679–1687. doi: 10.1053/j.gastro.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 106.Strnad P., et al. Keratin variants associate with progression of fibrosis during chronic hepatitis C infection. Hepatology. 2006;43:1354–1363. doi: 10.1002/hep.21211. [DOI] [PubMed] [Google Scholar]
- 107.Witt H., et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat. Genet. 2006;38:668–673. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamada T., et al. Combination therapy with an angiotensin-converting enzyme inhibitor and an angiotensin II receptor blocker synergistically suppresses chronic pancreatitis in rats. J. Pharmacol. Exp. Ther. 2005;313:36–45. doi: 10.1124/jpet.104.077883. [DOI] [PubMed] [Google Scholar]
- 109.Jaster R., Sparmann G., Emmrich J., Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579–584. doi: 10.1136/gut.51.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCarroll J.A., et al. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase (PI3-kinase) pathway. Biochem. Pharmacol. 2004;67:1215–1225. doi: 10.1016/j.bcp.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 111.Masamune A., Kikuta K., Satoh M., Kume K., Shimosegawa T. Differential roles of signaling pathways for proliferation and migration of rat pancreatic stellate cells. Tohoku J. Exp. Med. 2003;199:69–84. doi: 10.1620/tjem.199.69. [DOI] [PubMed] [Google Scholar]
- 112.Masamune A., et al. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J. Biol. Chem. 2002;277:141–147. doi: 10.1074/jbc.M107582200. [DOI] [PubMed] [Google Scholar]
- 113.Yoshida S., et al. Pancreatic stellate cells (PSCs) express cyclooxygenase-2 (COX-2) and pancreatic cancer stimulates COX-2 in PSCs. Mol. Cancer. 2005;4:27. doi: 10.1186/1476-4598-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCarroll J.A., et al. Vitamin A inhibits pancreatic stellate cell activation: implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89. doi: 10.1136/gut.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Talukdar R., Saikia N., Singal D.K., Tandon R. Chronic pancreatitis: evolving paradigms. Pancreatology. 2006;6:440–449. doi: 10.1159/000094561. [DOI] [PubMed] [Google Scholar]
- 116.Dornhofer N., et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816–5827. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 117.Aikawa T., Gunn J., Spong S.M., Klaus S.J., Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol. Cancer Ther. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 118.Gomez J.A., et al. Vitamin E attenuates biochemical and morphological features associated with development of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G162–G169. doi: 10.1152/ajpgi.00333.2003. [DOI] [PubMed] [Google Scholar]
- 119.Yoo B.M., et al. Novel antioxidant ameliorates the fibrosis and inflammation of cerulein-induced chronic pancreatitis in a mouse model. Pancreatology. 2005;5:165–176. doi: 10.1159/000085268. [DOI] [PubMed] [Google Scholar]
- 120.Shimizu K., et al. Thiazolidinedione derivatives as novel therapeutic agents to prevent the development of chronic pancreatitis. Pancreas. 2002;24:184–190. doi: 10.1097/00006676-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Van Westerloo D.J., et al. Therapeutic effects of troglitazone in experimental chronic pancreatitis in mice. Am. J. Pathol. 2005;166:721–728. doi: 10.1016/S0002-9440(10)62293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Emori Y., et al. Camostat, an oral trypsin inhibitor, reduces pancreatic fibrosis induced by repeated administration of a superoxide dismutase inhibitor in rats. J. Gastroenterol. Hepatol. 2005;20:895–899. doi: 10.1111/j.1440-1746.2005.03826.x. [DOI] [PubMed] [Google Scholar]
- 123.Su S.B., Motoo Y., Xie M.J., Taga H., Sawabu N. Antifibrotic effect of the herbal medicine Saiko-keishi-to (TJ-10) on chronic pancreatitis in the WBN/Kob rat. Pancreas. 2001;22:8–17. doi: 10.1097/00006676-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 124.Reding T., et al. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165–1173. doi: 10.1136/gut.2005.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoo B.M., et al. Amelioration of pancreatic fibrosis in mice with defective TGF-beta signaling. Pancreas. 2005;30:e71–e79.. doi: 10.1097/01.mpa.0000157388.54016.0a. [DOI] [PubMed] [Google Scholar]