Abstract
Drugs known as beta blockers, which antagonize the β-adrenergic receptor (β-AR), are an important component of the treatment regimen for chronic heart failure (HF). However, a significant body of evidence indicates that genetic heterogeneity at the level of the β1-AR may be a factor in explaining the variable responses of HF patients to beta blockade. In this issue of the JCI, Rochais et al. describe how a single amino acid change in β1-AR alters its structural conformation and improves its functional response to carvedilol, a beta blocker currently used in the treatment of HF (see the related article beginning on page 229). This may explain why some HF patients have better responses not only to carvedilol but to certain other beta blockers as well. The data greatly enhance our mechanistic understanding of myocardial adrenergic signaling and support the development of “tailored” or “personalized” medicine, in which specific therapies could be prescribed based on a patient’s genotype.
Chronic heart failure (HF) poses a major public health problem in this country, primarily due to the increasing proportion of our society surviving to the seventh and eighth decades of life and to improved treatment of acute ischemic cardiac events, resulting in more survivors who develop cardiac dysfunction. The group of drugs known as beta blockers block the effects of catecholamines, such as epinephrine and norepinephrine, on the body’s β-adrenergic receptors (β-ARs), slowing nerve impulses traveling through the heart and reducing the heart’s workload. While β-AR antagonists have become a mainstay of HF therapy, the most recent guidelines from the American College of Cardiology and the American Heart Association acknowledge that there are unresolved issues concerning the use of these drugs for the treatment of HF (1).
β1-AR polymorphisms and cardiac phenotype
The β-ARs are members of the G protein–coupled receptor (GPCR) superfamily, which consists of over 700 genes that are the targets of more than 50% of the drugs in clinical practice (2). There are 3 known types of β-ARs, β1, β2, and β3. The β1-ARs are located mainly in the heart, kidney, and adipose tissue. The β2-ARs are located mainly in the heart, lung, gastrointestinal tract, liver, pancreas, and skeletal muscle. The role and location of β3-ARs are less well defined. When stimulated by agonists, β-ARs primarily activate heterotrimeric guanine nucleotide–binding (G) proteins of the Gs family, causing dissociation of Gα-GTP and Gβγ subunits. The G proteins transduce intracellular signaling pathways via adenylyl cyclase (AC) activation, and this results in increased intracellular cAMP levels (3). This signaling cascade ultimately leads to positive regulatory input to the myocardial contractile apparatus. Importantly, β-ARs present on cardiomyocytes represent the most powerful means of enhancing the contractile performance of the heart.
The predominant β-AR subtypes of functional consequence to cardiac physiology are β1-AR and β2-AR, both of which have well-documented polymorphisms that exist in human populations. The coding region of the gene encoding the β1-AR, located on chromosome 10q24-26, contains 2 known SNPs resulting in amino acid substitutions. At position 49 in the extracellular amino terminus of the receptor, a serine is substituted by a glycine (Ser49Gly) with an allele frequency of 0.87 and 0.13, respectively (4). At position 389 in the intracellular carboxy terminus in the proximity of the seventh transmembrane spanning segment, a glycine or arginine can be found at an allele frequency of 0.25 and 0.75, respectively (4). These polymorphic variants of the β1-AR appear to have significant cardiovascular phenotypic consequences. For example, Ranade et al. found a significant functional association between the Ser49Gly polymorphism and resting heart rate, in which Gly49 homozygotes had the lowest heart rate, and each Ser49 allele increased the basal heart rate in an additive model (5). The Arg389 variant appears to be linked with HF in both clinical outcomes and response to therapy (6).
More specifically, the Arg389Gly polymorphism lies within the putative Gs-binding domain of the β1-AR (Figure 1), and consistent with this localization, previous in vitro studies in fibroblast cell lines transiently transfected with the Arg389 β1-AR have demonstrated enhanced receptor-Gs coupling as measured by 35S-GTP binding and slightly increased basal and markedly increased agonist-induced AC activity as compared with Gly389 β1-AR–transfected fibroblasts (7). Furthermore, in a study of transgenic mice with cardiac-specific overexpression of Arg389Gly β1-AR variants, Akhter and colleagues demonstrated that not only does Arg389 β1-AR lead to increased myocardial signaling properties, but it also confers cardioprotection following myocardial ischemia and reperfusion injury (8). This may involve the upregulation of GPCR kinase 2 (GRK2) activity, which was presumably induced by the increased signaling of the Arg389 variant, since the less active Gly389 form did not alter myocardial GRK2 levels.
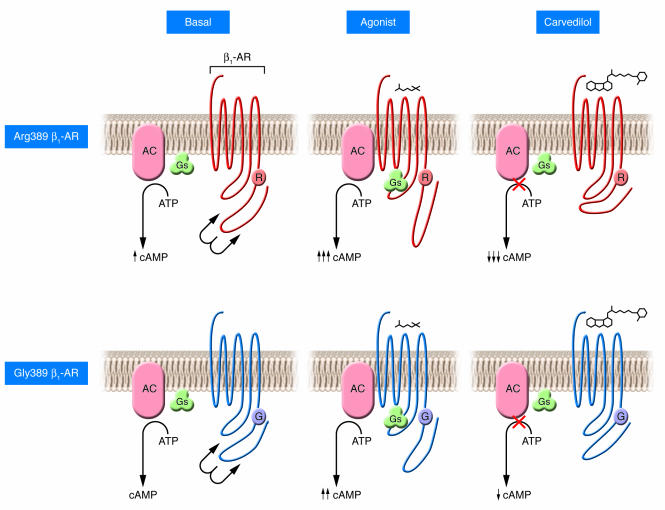
Figure 1. The effect of the β1-AR Gly389Arg polymorphism on signal transduction and beta blockade by carvedilol.
The Gly389Arg polymorphism of the β1-AR occurs in the region between the seventh transmembrane domain and the intracellular tail of the receptor (R and G correspond to arginine and glycine, respectively, at position 389 of the receptor). This highly conserved region is putatively associated with coupling to the Gs protein. The change of the amino acid residue at position 389 of the β1-AR from the polar, basic arginine (upper 3 panels) to the small, nonpolar glycine (lower 3 panels) may result in a modified structure that could alter receptor-Gs interaction. This region of the cytoplasmic tail of the receptor may participate in regulating the affinity of the receptor-Gs interaction. This could potentially explain the differences in receptor-Gs coupling and cAMP production associated with the Arg389 β1-AR variant as compared with the Gly389 β1-AR variant reported previously (7, 8). Under basal conditions, the Arg389 variant has been associated with increased cAMP production, which is markedly augmented as compared with the Gly389 variant following agonist stimulation (7). This effect could also be attributed to a more favorable structural environment for interaction with Gs. Carvedilol treatment induces a conformational change resulting in the intracellular tail of the receptor being positioned more closely to the third intracellular loop; this inverse agonism of the receptor in theory would lead to decreased Gs coupling and reduced cAMP accumulation. Both variants demonstrated inverse agonist properties in response to carvedilol; however, the effect was increased 2.5-fold in the Arg389 variant, apparently due to conformation of the Arg389 structure in the presence of the ligand as observed by Rochais et al. in this issue of the JCI (14).
In HF, the myocardium has a significant loss of contractile function. In order to compensate for the loss of systemic perfusion due to pump failure, the heart must increase heart rate and contractility. This is done by increased neurohumoral activation through increases in the activity of the sympathetic nervous system and renin-angiotensin system. Increases in the sympathetic catecholamines norepinephrine and epinephrine lead to chronic activation of myocardial β-ARs in order to drive the failing heart. However, chronic activation of these pathways, in particular activation of the β1-AR, leads to increased cardiotoxicity and cardiac pathology (3). This partially explains the clinical benefit achieved by β-AR antagonists, which block the overt noxious effects of catecholamines and have been shown to improve survival and reverse pathologic cardiac remodeling (9). Interestingly, sensitivity to the beneficial effects of β-AR blockade differs by ethnicity, as evidenced by data from the Beta-Blocker Evaluation of Survival Trial (BEST) demonstrating a lack of benefit in black patients with New York Heart Association class III or IV HF treated with the beta blocker bucindolol, compared with other patients (10). It was reasoned that racial differences in the incidence of HF and therapeutic response to β-AR antagonists may be heritable and that genetic heterogeneity with respect to β1-ARs may partially account for this phenomenon. However, conflicting data from clinical studies analyzing the association of β1-AR polymorphisms and response to β-AR antagonists have made it difficult to reach conclusions regarding the true clinical importance of β-AR polymorphisms with respect to treatment outcome (for review see ref. 11).
β1-AR signaling in real time
Recent advances in the fields of biochemistry and applied science have generated new classes of fluorescent probes to permit the direct assessment of the dynamic behavior of biological molecules. Specifically, fluorescence resonance energy transfer–based (FRET–based) techniques have allowed investigators to evaluate dynamic protein-protein interactions and protein conformational changes in real time (for reviews see refs. 12, 13). FRET fundamentally characterizes the spatiotemporal relationship of fluorescently labeled molecules within a system by way of recording the energy transfer from a donor fluorophore in an excited electronic state to an acceptor fluorophore of adequate proximity. In this issue of the JCI, Rochais and colleagues report on their exploitation of FRET technology to generate a β1-AR FRET sensor to directly assess the effects of the Arg389Gly polymorphism in the β1-AR on receptor activation, downstream signal transduction, and response to beta blockade in cultured human cells in real time (14). The β1-AR FRET sensor used in this study was generated based on evidence that the receptor undergoes conformational changes upon agonist binding, specifically in the region of the carboxy terminus and third intracellular loop, which move apart upon receptor activation. The authors then tagged these regions with cerulean and yellow fluorescent proteins, respectively, allowing them to detect the movement of these regions in real time using FRET, thereby tracking the dynamic changes in the receptor in response to agonist and antagonist binding. The authors demonstrated that this β1-AR FRET sensor shares pharmacological and signaling characteristics consistent with that of the WT β1-AR, including similar activation kinetics and downstream signaling via cAMP formation.
Utilizing β1-AR FRET sensors for both Gly389 and Arg389 variants of the β1-AR, the authors were able to directly assess the functional consequences of β-AR polymorphisms with respect to receptor conformation in response to pharmacologic agonists and antagonists. In contrast with results in previous in vitro studies (7, 8), the authors identified no significant differences between Gly389 and Arg389 variants in β1-AR–mediated Gs coupling or cAMP accumulation in response to agonist (14).
Perhaps the most exciting clinically relevant data from this study is the comparison of the FRET responses of the 2 β1-AR variants generated by the β-AR antagonists bisoprolol, metoprolol, and carvedilol. Each β-AR antagonist induced an increase in FRET ratio, signifying the occurrence of an active change in receptor conformation upon antagonist binding, suggesting an inverse agonist mechanism of action on the β-AR. Increases in FRET ratio were minor with respect to decreases in receptor activation for metoprolol and bisoprolol, and the effects of these 2 beta blockers did not differ between β1-AR variants (14). In contrast, carvedilol induced strong inverse agonism with regard to both β1-AR variants; furthermore, the Arg389 β1-AR variant exhibited a 2.5-fold increase in FRET ratio as compared with the Gly389 β1-AR variant. The unique properties of this beta blocker were confirmed downstream of receptor inactivation at the level of decreased cAMP accumulation: both β1-AR variants demonstrated marked reduction in basal cAMP levels (14). Carvedilol led to a much stronger reduction in basal cAMP content in the Arg389 β1-AR variant as compared with the Gly389 β1-AR variant (see Figure 1). The authors further established the phenotypic relevance of the molecular alterations induced by the Arg389Gly polymorphisms using primary neonatal rat cardiac myocytes infected with adenoviruses containing the β1-AR variants as a model system to study cardiac rate control. Under basal conditions, both β1-AR variants induced an increase in contractile activity as compared with WT cardiac myocytes; however, the contractile activity of the Arg389 variant was increased nearly 1.5-fold as compared with the Gly389 variant, and carvedilol significantly decreased this activity preferentially in the Arg389 β1-AR.
Overall, the study by Rochais et al. (14) represents the first direct assessment of GPCR function using the well-characterized β1-AR system that also happens to be critical for normal and pathological cardiac physiology. Of importance, the authors were able to assess the conformational changes that occurred during agonism and antagonism of the receptor. Moreover, the data reveal that different β1-AR antagonists, particularly carvedilol, are capable of inducing varying degrees of inverse agonism on the receptor and that this effect of carvedilol is dependent on the amino acid residue present at position 389 of the β1-AR. Carvedilol, unlike metoprolol or bisoprolol, is a nonselective beta blocker and can also act on α1-ARs (15). Therefore, it would be interesting to address the potential effects of β2-AR and α1-AR signaling on cardiac rate control to ascertain whether any receptor crosstalk or dimerization is responsible for the decreased basal cAMP production mediated by the Arg389 variant or whether antagonism of these other receptors is involved in the beneficial effects of carvedilol in HF. However, what is important to remember based on the current work is that for the β1-AR, carvedilol displays the greatest degree of inverse agonism, especially for the Arg389 variant (Figure 1), and studies such as this could lead to research broadening our knowledge of this pharmacological property of GPCR ligands and therefore potentially increase their clinical utility (16).
Taking it personally
The implications of these findings are of potentially profound clinical importance when considering the interindividual and ethnic variation that occurs in response to β-AR blocker therapy in the treatment of HF. It has been reported that the allele frequency of the Arg389 variant is 20% less common in black patients compared with non-black patients, and this may partially explain the poorer response to β1-AR antagonists seen in blacks compared with that of the rest of the population (17). Future efforts to characterize the effect of genetic heterogeneity in cellular response to pharmacological agents, such as the β1-AR antagonists examined in this study, lend credence to the viability of the concept of pharmacogenomics — pharmacologic intervention adapted according to an individual’s genetic makeup — and the realization of “personalized” medicine for the treatment of cardiovascular disorders such as HF (18).
Footnotes
Nonstandard abbreviations used: AC, adenylyl cyclase; β-AR, β-adrenergic receptor; FRET, fluorescence resonance energy transfer; GPCR, G protein–coupled receptor; HF, heart failure.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:86–89 (2007). doi:10.1172/JCI30476.
See the related article beginning on page 229.
References
- 1.Lee T.H.2001Management of heart failure: AHA/ACC guidelines summary. Heart disease: a textbook of cardiovascular medicine. 6th editio n . E. Braunwald, editor. 101652–685. [Google Scholar]
- 2.Becker O.M., et al. G-protein coupled receptors: in silico drug discovery in 3D. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11304–11309. doi: 10.1073/pnas.0401862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rockman H.A., Koch W.J., Lefkowitz R.J. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 4.Maqbool A., Hall S.A., Ball S.G., Balmforth A.J. Common polymorphisms of β1–adrenoreceptor: identification and rapid screening assay [research letter]. . Lancet. 1999;353:897. doi: 10.1016/s0140-6736(99)00549-8. [DOI] [PubMed] [Google Scholar]
- 5.Ranade K., et al. A polymorphism in the β1 adrenergic receptor is associated with resting heart rate. . Am. J. Hum. Genet. 2002;70:935–942. doi: 10.1086/339621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small K.M., McGraw D.W., Liggett S.B. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu. Rev. Pharmacol. Toxicol. 2003;43:381–411. doi: 10.1146/annurev.pharmtox.43.100901.135823. [DOI] [PubMed] [Google Scholar]
- 7.Mason D.A., Moore J.D., Green S.A., Liggett S.B. A gain-of-function polymorphism in a G-protein coupling domain of the human β1-adrenergic receptor. . J. Biol. Chem. . 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- 8.Akhter S.A., D’Souza K.M., Petrashevskaya N.N., Mialet-Perez J., Liggett S.B. Myocardial β1-adrenergic receptor polymorphisms affect functional recovery after ischemic injury. . Am. J. Physiol. Heart Circ. Physiol. . 2006;290: H1427–H1432. doi: 10.1152/ajpheart.00908.2005. [DOI] [PubMed] [Google Scholar]
- 9.Brauwald E., Bristow M.R. Congestive heart failure: fifty years of progress. Circulation. . 2002;102(20 Suppl. 4):IV14–IV23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 10.Beta-blocker Evaluation of Survival Trial Investigators. . A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. . N. Engl. J. Med. . 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 11.Leinweber K. Beta-adrenergic receptor polymorphism in human cardiovascular disease. Ann. Med. . 2004;36:64–69. doi: 10.1080/17431380410032544. [DOI] [PubMed] [Google Scholar]
- 12.Giempmans B.N., Adams S.R., Ellisman M.H., Tsien R.Y. The fluorescent toolbox for assessing protein localization and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 13.Miyawaki A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev. Cell. . 2003;4:295–305. doi: 10.1016/s1534-5807(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 14.Rochais F., et al. Real-time optical recording of β1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. . J. Clin. Invest. 2007;117:229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancy C.W., et al. Race and the response to adrenergic blockade with carvedilol in patients with chronic heart failure. N. Engl. J. Med. . 2001;344:1358–1364. doi: 10.1056/NEJM200105033441803. [DOI] [PubMed] [Google Scholar]
- 16.Bond R.A., Ijzerman A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol. Sci. 2006;27:92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Small K.M., Wagoner L.E., Levin A.M., Kardia S.L., Liggett S.B. Synergistic polymorphisms of β1- and α2c-adrenergic receptors and the risk of congestive heart failure. . N. Engl. J. Med. . 2002;347:1135–1142. doi: 10.1056/NEJMoa020803. [DOI] [PubMed] [Google Scholar]
- 18.Zineh I., Johnson J.A. Pharmacogenetics of chronic cardiovascular drugs: applications and implications. Expert Opin. Pharmacother. . 2006;11:1417–1427. doi: 10.1517/14656566.7.11.1417. [DOI] [PubMed] [Google Scholar]