Abstract
After intestinal injury, both the number and type of intestinal epithelial cells must be restored. Intestinal stem cells, located at the base of the intestinal crypt, repopulate the depleted crypt in a process known as compensatory proliferation. In this issue of the JCI, Brown et al. describe a new mechanism by which this process is regulated (see the related article beginning on page 258). Surprisingly, they find that a subset of stromal cells present within the intestinal tissue and expressing the proliferative factor prostaglandin-endoperoxidase synthase 2 (Ptgs2) is repositioned next to the intestinal stem cell compartment where local production of PGE2 controls injury-induced epithelial cell proliferation.
Most tissues of complex metazoans are renewable, which provides an obvious advantage for animals with an extended life span. In some tissues (for example, skin and intestinal epithelium), newly differentiated cells simply replace damaged and worn-out older cells. In the immune system, on the other hand, continuous replacement of lymphocytes is needed to maintain the diversity of antigen receptor specificities. The cell turnover rates vary widely among different tissues, with hematopoietic and intestinal epithelial cells, at the extreme end of the spectrum, characterized by the highest rate of renewal of as quick as 2–3 days. In all cases, tissue renewal is afforded by the presence of adult stem cells, which can produce new terminally differentiated cells in the steady state and as part of a tissue repair response triggered by injury-induced cell loss. The proliferative state of stem cells must therefore be regulated by extrinsic cues that report the tissue status and thus adjust the rate of tissue renewal. The nature of these signals, how they are regulated, and whether they act directly on stem cells or indirectly through the niche cells (cells neighboring stem cells and regulating their functions), is unknown in most cases. However, there is an enormous interest in answering these questions given their importance for understanding normal tissue homeostasis, tissue repair, and tumorigenesis.
The intestine as a model of tissue renewal and regeneration
The mammalian intestine is arguably the best model system with which to study the regulation of steady-state tissue renewal and the injury-induced tissue repair response. The small and large intestines of mammals are composed of cells from each of the 3 embryonic layers. The microarchitecture of the intestine consists of crypt-villous units (in the small intestine) and elongated crypts (in the colon). These units are lined with epithelial cells. The tops of the crypts and villi contain terminally differentiated cells, such as enteroabsorptive, goblet, and neuroendocrine cells. All of the differentiated intestinal epithelial cells arise from multipotent precursors, known as transit-amplifying cells, located closer to the bottom of the crypt. Hidden at the crypt base are the intestinal epithelial stem cells (Figure 1A).
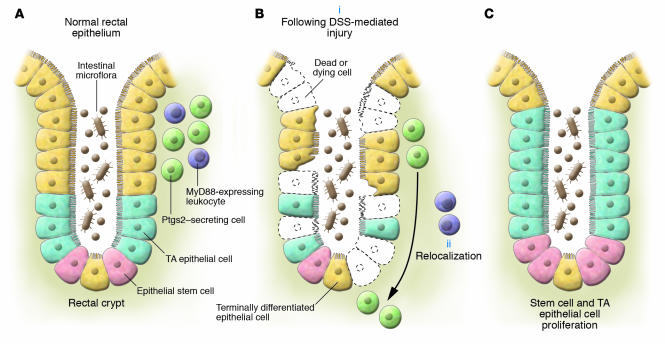
Figure 1. Model of MyD88-dependent relocalization of Ptgs2-expressing cells to the rectal crypt base and epithelial proliferation following DSS-induced injury.
(A) In the steady state, Ptgs2-expressing epithelial cells are mostly present in the lamina propria of the upper and middle regions of the rectal crypt. (B) Upon DSS-induced injury (i), these Ptgs2-expressing cells migrate to the bottom of the crypt, occupying a position near the stem cell niche (ii). This relocalization is dependent on MyD88 expression by leukocytes, presumably stimulated by TLR recognition of microbial products following barrier disruption. (C) Compensatory proliferation of stem cells and transit-amplifying (TA) epithelial cells after DSS-induced injury is dependent on MyD88 and Ptgs2, whereby a MyD88-dependent signal triggers repositioning of PGE2-producing cells to the crypt base, adjacent to the stem cell compartment.
In addition to epithelial cells, the intestinal tissue contains cells of mesenchymal origin, including leukocytes and fibroblasts. These cells are arranged in various locations throughout the crypts and villi, with the majority present in the lamina propria compartment of the mucosa below the epithelial basement membrane.
In the steady state, there is a modest amount of epithelial turnover in the intestine (1). At any given moment, there are a few epithelial cells dying at the top of the crypts. Physiologic cell loss is balanced by low-level, constant division of cells originating from the totipotent stem cells at the base of the crypt.
The proliferation of intestinal epithelial progenitor cells is also adaptable to circumstances under which there is a need to renew more than the steady-state number of cells. The intestinal epithelium plays an important role as a functional barrier and must be able to withstand widespread cell death due to ingestion of toxic compounds, microbial infection, inflammation, mechanical stresses, and iatrogenic causes including chemotherapy and radiation therapy.
Although tissue renewal and homeostasis are probably best understood for intestinal epithelium, many fundamental questions remain. How do intestinal epithelial cells know when to proliferate, i.e., how is injury perceived? Do these stimuli or signals of injury act directly on progenitor cells by converging on pathways known to regulate their proliferation (e.g., Wnt, bone morphogenic protein, Hedgehog, or Notch signaling pathways [ref. 2])? Or, alternatively, is injury perceived by mesenchymal cells, which in turn instruct progenitor cells to proliferate? The findings reported by Brown et al. (3) in this issue of the JCI add a surprising new dimension to these fundamental questions and suggest what we believe to be a novel mechanism for sensing tissue injury and initiating the tissue repair response in the colon.
Innate immune recognition of the commensal microflora as a cue for intestinal tissue repair
The lumen of mammalian intestine is home to trillions of microbes, predominantly bacteria, that encompass the indigenous commensal microflora (4). Recent studies with conventionally raised mice depleted of commensal bacteria (5) or germ-free mice (6, 7) administered the colonic epithelial toxin dextran sodium sulfate (DSS) have led to the conclusion that the microflora are required for protection from DSS-induced colonic injury and for induction of compensatory proliferation. Commensal bacteria contribute to tissue protection and repair by stimulating TLRs, which are best known for their essential functions in pathogen detection and protection from infection (8). Mice deficient in TLR2 and TLR4 (which recognize conserved bacterial products such as lipopeptides and LPS) or the TLR signaling adaptor myeloid differentiation factor 88 (MyD88) are phenotypically similar to germ-free mice with regard to protection from intestinal injury and subsequent compensatory proliferation (5, 7, 9, 10). Thus perception of injury and induction of tissue repair processes may occur via recognition of commensal microbial products that gain access to the TLRs expressed on myeloid cells upon epithelial barrier disruption (7, 11).
How then does TLR recognition of commensal bacteria lead to the induction of epithelial proliferation following injury? A number of studies implicated various growth factors in mediating epithelial regeneration after injury (12). Of these, PGE2, a biologically active and pleiotropic eicosanoid, has been shown to be particularly important for protection from colonic injury. PGE2 is a product of prostaglandin-endoperoxide synthase 2 (Ptgs2, also known as COX-2). Ptgs2-deficient mice show more colonic injury than WT controls upon DSS administration (13), and intraperitoneal injection of PGE2 increases epithelial proliferation after DSS exposure in WT mice (14). The TLR/MyD88 signaling pathway positively regulates Ptgs2 (15, 16), and in one study, isolated mesenchyme from DSS-treated descending colons revealed a MyD88-dependent induction of Ptgs2 mRNA (7). Based on these prior findings, it was reasonable to hypothesize that Ptgs2 may play a critical role in TLR/MyD88–dependent protection from epithelial injury in the colon.
In their studies, Brown, Stappenbeck, and colleagues (3) focused on the rectum, a region of the colon that is spared the severe injury that occurs upon DSS administration at the descending colon. They found that the rectal tissue of both Myd88–/– and Ptgs2–/– mice has similar defects in rectal crypt architecture and epithelial proliferation following injury compared with that of WT mice. The authors provide evidence that both MyD88 and Ptgs2 were part of the same signaling pathway, as administration of exogenous PGE2 to Myd88–/– mice rescued the defect in epithelial compensatory proliferation after injury.
Having established a functional relationship between MyD88 and Ptgs2 in the control of epithelial proliferation, Brown et al. (3) went on to investigate the mechanism of Ptgs2 regulation. It is here that things got intriguing. In contrast to what one would expect, the authors found that although rectal mesenchyme expresses Ptgs2 mRNA during the steady state, DSS administration did not increase Ptgs2 mRNA levels, though it did induce the expression of other genes. Interestingly, they identified what is believed to be a novel cell type in the rectal epithelial tissue that expresses Ptgs2 at high levels at the steady state and which the authors refer to as prostaglandin-expressing stromal cells (PSCs). Importantly, rectal injury did not lead to an increase in the number of PSCs or an increase in the rectal secretion of PGE2. Instead, DSS-induced epithelial injury led to a repositioning of the PSCs.
In the steady state, the majority of PSCs were located in the lamina propria lining the upper and middle third of the rectal crypt (Figure 1A). However, upon DSS-induced injury, the number of PSCs at these locales decreased and the number of PSCs increased at the crypt base, adjacent to the intestinal stem compartment (Figure 1B). The injury-induced relocalization of Ptgs2-expressing PSCs was absent in Myd88–/– mice and could be rescued by adoptive transfer of peripheral blood leukocytes from Rag1–/– (T cell– and B cell–deficient) mice, suggesting that MyD88 signaling in a cell derived from a nonlymphoid hematopoietic lineage, most likely the macrophage, is involved in the in situ repositioning of PSCs upon injury.
This observation of MyD88-dependent relocalization of PSCs to the crypt base (3), a region designated the “stem cell niche,” suggests that the local production of PGE2 by PSCs may be the mechanism by which MyD88 and Ptgs2 are linked in regulating rectal epithelial proliferation after injury. It remains to be determined whether the PGE2 produced by these relocated PSCs at the regenerating crypt base acts directly on epithelial progenitor cells to induce their proliferation (a PGE2 receptor, EP2, is expressed on regenerating epithelium in the small intestine [ref. 17]); acts on other cells, such as enteric neurons, that can trigger progenitor cell proliferation (18); or mediates an ancillary process such as angiogenesis (19) that may be required to support epithelial proliferation mediated by MyD88- or Ptgs2-independent pathways.
Further studies will undoubtedly reveal how MyD88 signaling in leukocytes leads to the movement of PSCs from the upper regions to the base of rectal crypts. TLR/MyD88 signaling induces many chemokines in addition to tissue remodeling factors such as MMPs. Thus, recruitment by chemoattraction and modulation of the extracellular matrix may be involved in the relocalization of PSCs during rectal injury. Identifying these MyD88-dependent processes will be an exciting avenue for future research. It would also be important to investigate whether the mechanism described by Brown et al. (3) operates in other parts of the colon and the small intestine.
TLR/MyD88 signaling is responsible for both transcriptional regulation of Ptgs2 (7, 20) and increased Ptgs2 protein expression by macrophages (20). In the proximal colon, Ptgs2 seems to play a primary role in negatively regulating the acute infiltration of leukocytes upon DSS-induced injury (13). Furthermore, while PGE2 seems to be important in small intestinal crypt survival and proliferation following radiation injury, this is due to COX-1 rather than Ptgs2 activity (21). It would be interesting to find out why different modes of regulation of PGE2 production have evolved and what aspects of the intestinal tissue physiology determine the choice of regulatory mechanism. It would also be worth examining whether a similar mechanism, based on repositioning of signal-emitting cells, is involved in the regulation of PGE2 production in other physiological and developmental settings. Like any other discovery, the findings by Brown et al. (3) point to many new exciting possibilities.
Footnotes
Nonstandard abbreviations used: DSS, dextran sodium sulfate; PSC, prostaglandin-expressing stromal cell; Ptgs2, prostaglandin-endoperoxide synthase 2.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:83–86 (2007). doi:10.1172/JCI30865.
See the related article beginning on page 258.
References
- 1.Marshman E., Booth C., Potten C.S. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 2.Radtke F., Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 3.Brown S.L., et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. . J. Clin. Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Rakoff-Nahoum S., Paglino J., Eslami-Varzaneh F., Edberg S., Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Kitajima S., Morimoto M., Sagara E., Shimizu C., Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp. Anim. 2001;50:387–395. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- 7.Pull S.L., Doherty J.M., Mills J.C., Gordon J.I., Stappenbeck T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. U. S. A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Araki A., et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 10.Fukata M., et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 11.Rakoff-Nahoum S., Hao L., Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Booth D., Potten C.S. Protection against mucosal injury by growth factors and cytokines. . J. Natl. Cancer Inst. Monogr. 2001;2001:16–20. doi: 10.1093/oxfordjournals.jncimonographs.a003433. [DOI] [PubMed] [Google Scholar]
- 13.Morteau O., et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J. Clin. Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessner T.G., Cohn S.M., Schloemann S., Stenson W.F. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. . Gastroenterology. 1998;115:874–882. doi: 10.1016/s0016-5085(98)70259-8. [DOI] [PubMed] [Google Scholar]
- 15.Rhee S.H., Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. . J. Biol. Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- 16.Eliopoulos A.G., Dumitru C.D., Wang C.C., Cho J., Tsichlis P.N. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. Embo J. 2002;21:4831–4840. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houchen C.W., Sturmoski M.A., Anant S., Breyer R.M., Stenson W.F. Prosurvival and antiapoptotic effects of PGE2 in radiation injury are mediated by EP2 receptor in intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G490–G498. doi: 10.1152/ajpgi.00240.2002. [DOI] [PubMed] [Google Scholar]
- 18.Bjerknes M., Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. . Proc. Natl. Acad. Sci. U. S. A. 2001;98:12497–12502. doi: 10.1073/pnas.211278098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D., et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukata M., et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houchen C.W., Stenson W.F., Cohn S.M. Disruption of cyclooxygenase-1 gene results in an impaired response to radiation injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G858–G865. doi: 10.1152/ajpgi.2000.279.5.G858. [DOI] [PubMed] [Google Scholar]