Abstract
Objectives. We sought to determine the extent of HIV testing among urban injection drug users (IDUs) to assess whether an expansion of targeted testing programs would be consistent with national goals to identify previously undetected infections.
Methods. IDUs in 5 US cities (Oakland, Calif; Chicago, Ill; Hartford and New Haven, Conn; and Springfield, Mass) were recruited either by chain referral or time–location sampling. The IDUs were questioned about HIV testing, and factors associated with HIV testing were analyzed.
Results. Ninety-three percent of 1543 IDUs had been tested. Among those tested but who did not report having been told that they were HIV seropositive, 90% had been tested within the past 3 years. Women and syringe-exchange customers were more likely to have been tested ever and in the recent past. We estimated the number of undetected infections among urban IDUs in the United States to be less than 40000.
Conclusions. Testing for HIV has reached the vast majority of IDUs through the current options. Expending scarce prevention money to expand testing of IDUs is unlikely to be productive. Instead, resources should be used for proven HIV-prevention strategies including syringe exchange, drug treatment, and secondary prevention for those who are HIV positive
The Centers for Disease Control and Prevention (CDC) has estimated that there are between 850 000 and 950 000 people living with HIV in the United States.1 It is further estimated that there have been about 40000 new infections and about 40000 new cases of AIDS yearly since 1997.2 The introduction of effective antiretroviral therapy in the mid-1990s has had an enormous impact on the epidemic, decreasing the number of deaths attributed to AIDS to between 10000 and 20000 annually since 1997. Thus, the number of people living with HIV and AIDS has been steadily increasing. Unfortunately, there appears to be a testing gap that is leaving many people who are infected with HIV unaware of their status for long periods during which they may transmit the virus to others. Current estimates are that approximately 1 in 4 of those infected with HIV, as many as a quarter of a million people, do not know that they are infected.1
Among the groups at highest risk for HIV infection are injection drug users (IDUs). Data from the CDC indicate that slightly more than one quarter of the 920 000 AIDS cases diagnosed through 2003 were attributed solely to injection drug use.3 For the last 3 years with available data (2001–2003), however, the percentage of AIDS diagnoses and HIV infections attributable to injection drug use has been declining. New AIDS cases attributable solely to injection drug use comprised 23.1% of the total newly reported AIDS cases; new HIV diagnoses comprised only 16.1% of the total new HIV diagnoses, although this number may be an underestimate because many locations with large numbers of IDUs have not yet instituted named HIV reporting.3 Nevertheless, HIV prevalence among IDUs remains high. Data from 16 sites nationwide found the overall prevalence was 12.7%.4 Testing of IDUs for HIV infection is important for surveillance and prevention. Given 2 recent policy shifts, it is equally important to document whether a testing gap exists for IDUs.
The first shift is a new CDC strategy for HIV prevention first announced in the spring of 2004.5 This new approach, “Advancing HIV Prevention,” is part of an initiative to promote early detection of those who are infected with HIV, to enhance their referral into care, to focus prevention on persons living with HIV, and to expand prevention efforts for persons at high risk of becoming infected. Testing for HIV is a central feature of the initiative. The CDC envisions expanded testing as the first step in reducing transmission and bringing the infected into care. It based this decision on the high percentage of high-risk individuals who are infected but have not been tested, are therefore unaware of their HIV serostatus, and are more likely than those diagnosed to engage in unsafe sex.6–9
The second shift is toward routine testing for HIV. Impetus for this comes from a pair of articles and an editorial published in 2005 in the New England Journal of Medicine.10–12 Paltiel et al. used a cost-effectiveness analysis to demonstrate that expanded testing would have its greatest impact for the least cost when provided to high-risk populations with unknown HIV prevalence in excess of 3% and when such individuals are tested once every 3 years.11 Life expectancies for those with HIV infection would be increased at costs well within the bounds established for cost-effective care. An alternative set of recommendations was made by the US Preventive Services Task Force, which emphasized expanded testing for high-risk groups rather than routine testing.13
Implicit in the calls for routine testing and expansion of testing for high-risk groups is the assumption that the percentage of high-risk individuals who do not know that they are infected is high. However, before the CDC expends its scarce resources on testing programs targeted to IDUs, it is important to determine whether expanded testing of IDUs will be effective in identifying more IDUs infected with HIV. We analyzed data collected from IDUs in 5 cities—Hartford and New Haven, Conn; Springfield, Mass; Chicago, Ill; and Oakland, Calif—to examine the extent of HIV testing among active IDUs and identify factors associated with testing and test results. These data can prove useful in determining how the CDC should implement its “Advancing HIV Prevention” program.
METHODS
Study Sites and Participants
We combined data from 2 studies for this analysis. The first study—the Diffusion of Benefit From Syringe Exchange study—was conducted in Hartford, Chicago, and Oakland, and investigated the extent to which safe injection knowledge and equipment diffused from syringe exchanges to the broader community of IDUs.14–16 The second study—the Syringe Access, Use, and Discard study—was conducted in Hartford, New Haven, and Springfield to determine the influence of syringe access, use, and discard practices on HIV-related risk behaviors.16–18
Both studies recruited active IDUs. Enrollment criteria included a report of any injection of illegal drugs in the month before enrollment and either visual evidence of injection stigmata or demonstrable knowledge of drug injection techniques. The studies were approved in advance by the institutional review boards of all participating institutions.
For the Diffusion of Benefit From Syringe Exchange study, participants were recruited by chain referral starting from active IDUs who were customers of their local syringe-exchange program (SEP). A total of 560 individuals were recruited into this study at baseline between November 1998 and October 2000. About 20% of the study participants were customers of the SEP.14
For the Syringe Access, Use, and Discard study, participants were recruited by targeted sampling designed to enroll at least 40 active IDUs in each of 8 neighborhoods in each of the 3 cities. Eligible individuals were identified by intensive street outreach in each neighborhood. A total of 983 individuals were interviewed between September 2000 and March 2002.
Data Collection Instruments
All individuals recruited into these studies were interviewed after providing informed consent. The interviews were primarily structured and were based on the National Institute on Drug Abuse HIV Risk Behavior Assessment.19 Our modifications to this instrument have been discussed previously.15–17 For this analysis, we used a series of questions about HIV status and history of HIV testing. Questions included “Have you ever had a test for HIV (the AIDS virus)?”; “If yes, how many times?”; “When were you last tested for HIV?”; and “Have you ever been told by a healthcare worker that you are HIV-positive?”
Data Analysis
Data sets from the Diffusion of Benefit From Syringe Exchange and Syringe Access, Use, and Discard studies were merged. Because both studies independently recruited participants in Hartford, we used a common code (containing initials, gender, and date of birth) to identify and remove 29 duplications from the database. In the case of duplicates, the more recent data were used in the analysis.
Data were analyzed using SPSS, version 12 (SPSS Inc, Chicago, Ill). History of HIV testing and self-reported prevalence were analyzed to determine whether these items differed as a function of city, sociodemographic characteristics, and injection and sexual risk behaviors. Injection risk behaviors included injection frequency, sharing of syringes or other injection paraphernalia, and various forms of sharing drugs with other IDUs.20,21 Sexual risk behaviors included number of recent sexual partners and inconsistent condom use. Where noted, continuous and multicategory variables were dichotomized.
Bivariate analyses were performed with ever-tested, recently tested (within the past 1 or 3 years), and self-reported HIV status as the dependent variables. For bivariate analysis of dichotomous variables, we ran tests and reported dependent variables associated at a level of P ≤ .05. For variables with more than 2 categories, we ran unconditional logistic regression. For self-reported HIV status, we calculated and reported odds ratios with 95% confidence intervals. A logistic regression model was constructed with city as the proximate factor predicting seroprevalence, and all other significant variables entered as potential covariates.
RESULTS
Testing of Urban IDUs
Merging the 2 studies yielded a total of 1543 individuals, all but 2 of whom responded to the question of ever having been tested for HIV. The study population was 65.2% male (n = 1006) and mostly minority (n = 1227; 37.2% African American and 42.7% Hispanic). The mean and median ages were 39.7 ± 8.4 years and 39 years, respectively. Slightly more than half of respondents (n = 832; 54.1%) reported completing high school. Heroin, cocaine, and methamphetamine injection in the 30 days before interview was reported by 1495, 714, and 22 respondents, respectively. Syringe-exchange programs were used at least once within the 30 days before interview by 30% of respondents (n = 464), although syringe exchange was not available in Springfield.
Having ever been tested for HIV was reported by 1435 (93.0%) respondents. The mean, median, and modal numbers of times tested per individual were 4.9, 3, and 2, respectively. When we examined sociodemographic variables, all groups were tested at rates higher than 90%. Nevertheless, significant differences (P < .05) were detected for gender and for ethnicity. More women reported having been tested (95.5%) than men (91.8%). Among the 3 major racial/ethnic groups, Whites were most likely to have been tested (95.7%); Hispanics were least likely (91.5%). Neither the types of drugs injected nor the risk behavior variables were associated with the likelihood of ever having been tested for HIV. Customers of SEPs were significantly more likely to have been tested (96.1%) than were noncustomers (91.8%). Chicago was the only city in which less than 90% of IDUs reported having been tested (Table 1 ▶). The rate of testing was significantly different among the cities (P < .05). All of these factors remained significant in a logistic regression analysis (Table 2 ▶).
TABLE 1—
Self-Reported HIV Testing and Prevalence Among Urban Intravenous Drug Users in 5 Cities: 1998–2002
| Oakland, Calif n = 163 | Chicago, Ill n = 289 | Hartford, Conn n = 440 | New Haven, Conn n = 320 | Springfield, Mass n = 331 | |
| Ever tested, n (%) | 151 (92.6) | 255 (88.2) | 418 (95.0) | 300 (93.8) | 306 (92.4) |
| HIV+, n (% of ever tested) | 4 (2.7) | 27 (10.6) | 102 (24.4) | 69 (23.0) | 98 (32.0) |
| Seronegative, na | 147 | 228 | 316 | 231 | 208 |
| Tested previous year, n (%)b | 110 (74.8) | 159 (69.7) | 248 (78.5) | 139 (60.1) | 120 (57.7) |
| Tested previous 3 years, n (%)b | 131 (89.1) | 213 (93.4) | 296 (93.7) | 200 (86.6) | 172 (82.7) |
aThe number of intravenous drug users who reported being tested for HIV and did not report being told that they were HIV seropositive.
bThe number and percent among tested, non–HIV-positive intravenous drug users.
TABLE 2—
Multivariate Analysis of Factors Associated With HIV Testing of Urban Intravenous Drug Users in 5 Cities: 1998–2002
| Odds Ratio | 95% CI | |
| Gender | ||
| Male | Referent | |
| Female | 1.95 | 1.20, 3.15 |
| Race/ethnicity | ||
| Hispanic | Referent | |
| Non-Hispanic White | 2.29 | 1.17, 4.48 |
| African American | 1.56 | 0.98, 2.50 |
| SEP customer | ||
| No | Referent | |
| Yes | 2.22 | 1.26, 3.89 |
| City | ||
| Chicago, Ill | Referent | |
| Hartford, Conn | 2.33 | 1.27, 4.24 |
| New Haven, Conn | 2.44 | 1.34, 4.44 |
| Oakland, Calif | 1.09 | 0.53, 2.25 |
| Springfield, Mass | 1.78 | 0.93, 3.43 |
Notes. CI = confidence interval; SEP = syringe-exchange program.
For the 1130 individuals who reported having been tested but who did not report having been told that they were HIV seropositive, we investigated the timing of their most recent HIV test (Figure 1 ▶). More than two thirds (n = 776, 68.7%) had been tested within the past year and 720 (92.8%) of these individuals reported that their recent test was not their first. Nearly 90% (n = 1012, 89.6%) had been tested within past 3 years, and 921 (91.0%) reported that their most recent test was not their first time tested. The distribution in the numbers of tests for these individuals revealed that repeat testing was the norm for this population of IDUs. The mean and median number of times tested were 4.87 (SD= 6.59) and 4. Modal responses were 2 and 3. A total of 160 people reported 10 or more HIV tests.
FIGURE 1—
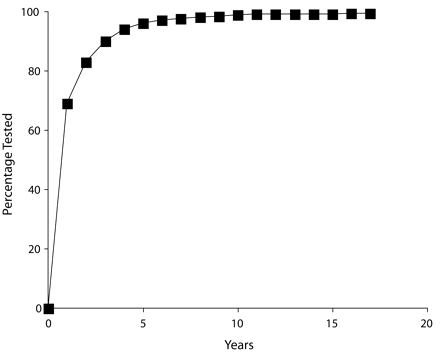
Proportion of injection drug users (IDUs) who had received an HIV test and had never been informed of an HIV seropositive diagnosis, by years since last HIV test: Hartford, Conn; New Haven, Conn; Oakland, Conn; Springfield, Mass; and Chicago, Ill; 1998–2002.
Note. A total of 1130 IDUs reported being tested and did not report having been told that they were infected with HIV. The cumulative frequency of the percentage of these individuals who reported having been tested within a given number of years is represented.
We investigated the factors associated with individuals being tested more recently, both within the past year and within the past 3 years. Women were more likely than men to have had a test either within the past year or within the past 3 years. In a bivariate analysis by age, we compared those aged 39 years or younger with those aged older than 39 years and found that those participants aged 39 years or younger were significantly more likely to have been tested. SEP customers were more likely to have been tested than noncustomers. Neither the types of drugs injected nor any of the injection-related or sexual risk behaviors were associated with recent testing.
Less than one fifth of the respondents (n = 300, 19.0%) reported being HIV seropositive. Prevalences of HIV were higher in the cities of the northeast, with the highest prevalence reported in Springfield (Table 1 ▶). These results are in keeping with expectations, based on prevalence rates reported in past studies.4,22,23 Male gender, race other than non-Hispanic White, older age, and being less well educated were the only factors significantly associated with reporting being HIV seropositive. Among the injection-related risk behaviors, sharing of syringes, water, or paraphernalia was not associated with reporting being HIV seropositive, but injection frequency and syringe-mediated drug sharing (i.e., use of syringes to divide or apportion drugs) were. Consistent condom use was significantly associated with being seropositive, a finding that might reflect the adoption of preventive behaviors once individuals are advised that they are infected. When all the significant variables were combined in a logistic regression analysis that first entered city of residence, only city, older age, and consistent condom use remained significantly associated with a report of being HIV seropositive.
Estimating Undetected Infections
For the population of urban IDUs in the United States, it is possible to estimate the prevalence of undetected infections (Figure 2 ▶). It has been estimated that there are slightly more than 1.3 million urban IDUs.24 Three sources of infections unknown to the person who is infected exist: those prevalent among the untested population, those prevalent among tested IDUs who were not informed of their status, and incident infections occurring among IDUs since their most recent test.
FIGURE 2—
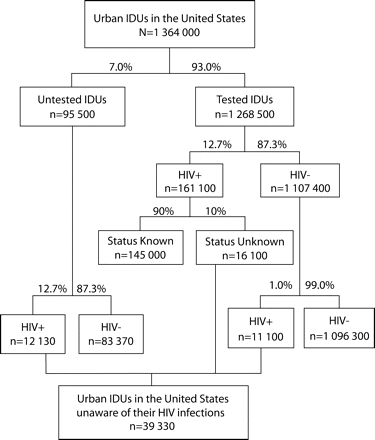
Number of undetected infections among urban intravenous drug users (IDUs) in the United States.
Note. Starting with an estimate for the number of intravenous drug users in the 96 largest urban areas,24 the number of undetected infections among untested and previously tested IDUs is estimated.
To estimate prevalent infections in the untested population, we began with the 7% of the participants in our study who had never been tested. To estimate the prevalence in this population, we used a figure of 12.7% HIV seropositive, a figure that comes from the largest nationwide study of IDUs, the National Institute on Drug Abuse–funded Cooperative Agreement Consortium.4 This resulted in an estimate of 12130 HIV-infected individuals among this group.
To estimate the number of IDUs who have tested positive but have not been informed of their results, we began with the estimated 161000 tested IDUs who were HIV positive at a prevalence of 12.7%. Unfortunately, it is hard to estimate the percentage of IDUs who failed to return for their results because the tests were conducted at a variety of different locations, and the rate at which IDUs were informed of their status could have varied tremendously. Certainly, in those contexts in which IDUs were in continued contact with the site doing the testing (e.g., prisons, methadone programs, long-term abstinence-based drug treatment programs, syringe exchange programs, and during pregnancy and in-patient hospitalizations) the rate at which IDUs would be informed of their status would be higher than the rates observed at voluntary counseling and testing sites or as part of a research study. Return rates of 81% were reported for one research study25 whereas a return rate of 93% was reported for IDUs entering substance-abuse treatment.26
Given the large percentage of IDUs who reported having been in treatment or in prison, we might guess that 90% of IDUs end up being told their test results, but a better estimate depends on obtaining more information about where IDUs get tested and what percentage of IDUs are informed at those sites. Therefore we have used a figure of 10% to estimate the number of IDUs who were tested and found to be HIV positive but who had not been made aware of their positive serostatus. Thus, we estimated that there were 16100 IDUs who have been tested, were found to be infected, and have not been made aware of their status.
To estimate incident infections among those who previously tested negative, we used data from studies from around the United States, including those conducted for periods since 1995 in the San Francisco Bay Area, Chicago, Baltimore, New York, New Haven, and Rhode Island.27–34 The mean incidence rate from these studies was 1.2 new infections per 100 person-years, with a range between zero and 1.8 new infections per 100 person-years. We can estimate the number of undetected infections among those previously tested by multiplying this average incidence rate by the percentage of people at risk, using the data presented in Figure 1 ▶. This estimate can be expressed as
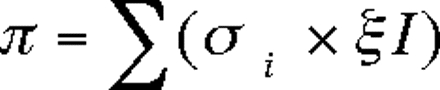
where π is the prevalence, si is the percentage of individuals in each year who have not been told they are infected and not been tested in that year, and I is the incidence rate.
For example, for individuals not aware of being positive, the prevalence of infection among this population as a result of having been infected in the past year would be estimated at 0.31 × 0.012=0.0037. Likewise, for the 17% not tested between 1 and 2 years before interview, the rate of infection would be estimated at 0.17 × 0.012=0.0020. Summing over 20 years yielded an estimate for undetected HIV infections of 1.0% among tested IDUs who have not been told they are HIV positive. For the 93% who had been tested, 87.3% remain susceptible, yielding about 1.1 million such individuals. If the prevalence in this population was 1.0% as estimated earlier, this would add another 11100 people to the ranks of the undetected infections.
We combined the 3 estimates—the untested, those tested but not informed by a healthcare worker that they are HIV positive, and those infected since last tested—which yielded an estimate of 39330 undetected HIV infections among urban IDUs in the United States.
DISCUSSION
We have shown evidence that HIV testing of 1 population at high risk for HIV infection—urban IDUs—has achieved substantial coverage. More than 90% of the urban IDUs interviewed in 5 US cities have been tested. This percentage is an improvement over the rates of 73% and 81% observed in the samples of IDUs accrued in the early 1990s and somewhat higher than the 83.9% rate observed among IDUs in Baltimore.26,35,36
We also found that for those tested but not informed of being HIV infected, 90% had been tested within 3 years. A sizable majority of these individuals also reported that they have been repeatedly tested. Qualitative studies have suggested that these individuals are cognizant that they are at risk for HIV infection and that they view testing in a fashion similar to screening for a chronic illness.37 Given that in previous studies women were more likely to report health and family reasons for being tested,38 it is interesting to note that in our study women were both more likely to have been tested and to have been tested recently. A similar finding, that women are more likely to have been tested, was observed in the multisite National Institute on Drug Abuse National AIDS Research Consortium,39 but the finding is not universal.40
There are several limitations to this study. First, HIV prevalence is self-reported. We have no way to know the accuracy of the reports, although we could find no studies reporting that IDUs tend to misreport their HIV status.
Second, we do not know at what venues or under what circumstances individuals were tested and what percentage of the people tested did not obtain the results of their most recent HIV test. We provided a crude estimate for the number of IDUs who have been tested, were found to be HIV positive, and have not received their test results. Individuals in this category would need to be retested. Studies assessing failure to return for test results found associations with lower educational achievement, being younger, engaging in commercial sex work, and previous imprisonment.25 On the other hand, imprisoned individuals are generally tested and informed of the results. Regardless of the reason, such failure to obtain one’s test result would blunt the effectiveness of testing. Rapid testing might improve testing rates—several studies have indicated that rapid testing would be preferred by IDUs, if problems with false-positive tests could be overcome.41–43 Although they are more expensive on a per-test basis, they appear to be cost-effective in terms of providing diagnoses to HIV-positive individuals.44
Third, the data come only from urban IDUs. Intravenous drug users from suburban or rural communities may be less likely to get tested for HIV, but, on the other hand, HIV prevalence among nonurban IDUs seems to be lower than among their urban counterparts.45
By our estimates, there are fewer than 40000 undetected HIV infections among urban IDUs. Furthermore, more recent findings suggest that prevalence rates among IDUs are declining, especially in previously high-prevalence regions such as New York and the northeast.3,34,46,47 Lower incidence rates among those who tested negative would reduce the number of undetected infections even further.
The new CDC HIV-prevention strategy is predicated on the belief that approximately one quarter of a million Americans are unaware that they are infected with HIV1 and that one quarter of individuals in high-risk groups are unaware of their HIV status.5 For urban IDUs, our findings revealed that this second belief is unfounded. Less than one tenth of urban IDUs have not been tested and fewer than one eighth of all individuals estimated to be infected without knowing it are urban IDUs. Although these numbers are not insignificant, the high rates of recent and repeat testing for urban IDUs at risk for HIV infection suggest that little additional prevention money should be expended to specifically target this population unless strategies can be employed that identify and test specifically the previously untested.
As perhaps the only exception to this blanket statement that targeting IDUs is unnecessary and unproductive, one might consider programs designed to reach and test Hispanic IDUs. In our study, Hispanic IDUs are least likely to have been tested and most likely to be HIV infected.
If expanding testing to identify HIV-positive individuals is the first step in a multifaceted approach to expanding HIV prevention, and this approach is unlikely to be either productive or cost-effective for populations of urban IDUs, then it is important to determine what steps would be most likely to keep incidence rates low. As the history of the HIV epidemics among IDUs has demonstrated, epidemics can erupt even when low prevalence has been the norm for a long time. In Vancouver, low prevalence and a large, well-established SEP did not prevent an outbreak when the drug of choice changed and the need for more syringes could not be met by the SEP.48,49 The epidemic of HIV among IDUs in the former Soviet Union occurred despite the existence of an extensive, nationwide testing system that conducted tens of millions of HIV tests annually.50,51 Keeping incidence low will require expanding programs such as SEPs and clinically proven drug-treatment services that have been amply demonstrated to prevent HIV transmission in a cost-effective manner.52–54
Our finding that HIV testing was higher among SEP customers provides an additional rationale for increasing funding to SEPs. Expanding antiretroviral therapy to urban IDUs is necessary, but this will require novel approaches to promote access and adherence.55–57 Recent reports have made it clear that even though IDUs have benefited from antiretroviral therapy commencing when CD4 cell counts have fallen to levels that result in an AIDS diagnosis, it may be more appropriate to consider initiating therapy once CD4 cell counts fall below 350 per μL.58,59 It is in these proven directions that scarce prevention resources should be allocated.
Acknowledgments
Funding for the Diffusion of Benefit from Syringe Exchange Programs study was provided by a grant co-funded by the National Institute on Drug Abuse and the National Institute of Mental Health (grant P01-MH56826). Funding for the Syringe Access, Use, and Discard Study was provided by the National Institute on Drug Abuse (grant R01-DA12569). Summer support for Erin Curtin was provided by a research internship from the Yale Center for Interdisciplinary Research on AIDS funded by the National Institute of Mental Health (grant P30-MH62294).
Human Participant Protection Research protocols and consent documents for the 2 projects were fully reviewed and approved before the start of data collection by institutional review boards at all institutions that participated in the studies.
Peer Reviewed
Contributors R. Heimer was the principal investigator for the Diffusion of Benefit from Syringe Exchange Study, originated the analysis, and wrote most of the article. L. E. Grau was project director for the Diffusion of Benefit from Syringe Exchange Study, participated in the design of the data analysis, cleaned and merged the data, and conducted many of the analyses. E. Curtin assisted R. Heimer and L. E. Grau in conducting refinements of the initial analyses. K. Khoshnood participated in the design of the data analysis, edited the article, and suggested additional analyses. M. Singer was the principal investigator for the Syringe Access, Use, and Discard study, participated in writing, and suggested refinements to interpretation of the analyses.
References
- 1.Fleming P, Byers RH, Sweeney PA, Daniels D, Karon JM, Janssen RS. HIV prevalence in the United States, 2000. Paper presented at: 9th Conference on Retroviruses and Opportunistic Infections; February 25, 2002; Seattle, Wash.
- 2.Janssen RS, Onorato IM, Valdiserri RO, et al. Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;52:329–332. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2003. Atlanta, Ga: US Dept of Health and Human Services; 2004.
- 4.Kral AH, Bluthenthal RN, Booth RE, Watters JK. HIV seroprevalence among street-recruited injection drug and crack cocaine users in 16 US municipalities. Am J Public Health. 1998;88:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Division of HIV/AIDS Prevention Centers for Disease Control and Prevention. Advancing HIV Prevention: New Strategies for a Changing Epidemic. Available at: http://www.cdc.gov/hiv/partners/AHP-brochure.htm. Accessed July 15, 2005.
- 6.Anderson JE, Carey JW, Taveras S. HIV testing among the general US population and persons at increased risk: information from national surveys, 1987–1996. Am J Public Health. 2000;90:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HIV Testing Survey. HIV/AIDS Special Surveillance Report 5. Atlanta, Ga: Centers for Disease Control and Prevention; 2002.
- 8.Kellerman SE, Lehman JS, Lansky A, et al. HIV testing within at-risk populations in the United States and the reasons for seeking or avoiding HIV testing. J Acquir Immune Defic Syndr. 2002;31:202–210. [DOI] [PubMed] [Google Scholar]
- 9.Marks G, Crepaz N, Senterfritt W, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39: 446–453. [DOI] [PubMed] [Google Scholar]
- 10.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. New Engl J Med. 2005;352:570–585. [DOI] [PubMed] [Google Scholar]
- 11.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States—an analysis of cost-effectiveness. New Engl J Med. 2005; 352:586–595. [DOI] [PubMed] [Google Scholar]
- 12.Bozzette SA. Routine screening for HIV infection—timely and cost-effective. New Engl J Med. 2005;352: 620–621. [DOI] [PubMed] [Google Scholar]
- 13.US Preventive Services Task Force. Screening for HIV: recommendation statement. Ann Intern Med. 2005;143:32–37. [DOI] [PubMed] [Google Scholar]
- 14.Heimer R, Clair S, Grau LE, Bluthenthal RN, Marshall PA, Singer M. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction. 2002;97: 1277–1287. [DOI] [PubMed] [Google Scholar]
- 15.Bluthenthal RN, Malik MR, Grau LE, Singer M, Marshall PA, Heimer R. Sterile syringe access conditions and variations in HIV risk among drug injectors in three cities. Addiction. 2004;99:1136–1146. [DOI] [PubMed] [Google Scholar]
- 16.Heimer R, Clair S, Teng W, Grau LE, Khoshnood K, Singer M. Effects of increasing syringe availability on syringe-exchange use and HIV risk: Connecticut 1990–2001. J Urban Health. 2002;79:556–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchanan D, Shaw S, Teng W, Hiser P, Singer M. Neighborhood differences in patterns of syringe access, use, and discard among injection drug users: implications for HIV outreach and prevention education. J Urban Health. 2003;80:438–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer M, Stopka T, Shaw S, et al. Lessons from the field: from research to application in the fight against AIDS among injection drug users in three New England cities. Hum Organ. 2005;64:179–191. [Google Scholar]
- 19.National Institute on Drug Abuse. Risk Behavior Assessment. Rockville, MD: National Institutes of Health, Dept of Health and Human Services; 1991.
- 20.Grund JP, Kaplan CD, Adriaans NF, Blanken P, Huisman J. The limitations of the concept of needle sharing: the practice of frontloading. AIDS. 1990;4: 819–821. [PubMed] [Google Scholar]
- 21.Koester S, Glanz J, Baron A. Drug sharing among heroin networks: implications for HIV and hepatitis B and C prevention. AIDS Behav. 2005;9:27–39. [DOI] [PubMed] [Google Scholar]
- 22.Watters JK, Bluthenthal RN, Kral AH. HIV seroprevalence in injection drug users. JAMA. 1995;273: 1178. [PubMed] [Google Scholar]
- 23.Monterroso ER, Hamburger ME, Vlahov D, et al. Prevention of HIV infection in street-recruited injection drug users. The Collaborative Injection Drug User Study (CIDUS). J Acquir Immune Defic Syndr. 2000;25: 63–70. [DOI] [PubMed] [Google Scholar]
- 24.Friedman SR, Tempalski B, Cooper H, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004;81: 377–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziek K, Goldstein MF, Beardsley M, Deren S, Tortu S. Factors associated with HIV testing and returning for test results in a sample of out-of-treatment drug users. J Drug Issues. 2000;30:675–686. [Google Scholar]
- 26.Samet JH, Mulvey KP, Zaremba N, Plough A. HIV testing in substance abusers. Am J Drug Alcohol Abuse. 1999;25:269–280. [DOI] [PubMed] [Google Scholar]
- 27.Kral AH, Lorvick J, Gee L, et al. Trends in human immunodeficiency virus seroincidence among street-recruited injection drug users in San Francisco, 1987–1998. Am J Epidemiol. 2003;157:915–922. [DOI] [PubMed] [Google Scholar]
- 28.Ouellet LJ, Thorpe LE, Huo D, et al. Prevalence and incidence of HIV among out-of-treatment injecting drug users, Chicago 1994–1996. J Acquir Immune Defic Syndr. 2000;25:443–450. [DOI] [PubMed] [Google Scholar]
- 29.Nelson KE, Galai N, Safaeian M, Strathdee SA, Celentano DD, Vlahov D. Temporal trends in the incidence of human immunodeficiency virus infection and risk behavior among injection drug users in Baltimore, Maryland, 1988–1998. Am J Epidemiol. 2002;156: 641–653. [DOI] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Diaz T, Perlis T, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. Am J Epidemiol. 2003;157:467–471. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EH, Heimer R. HIV incidence among New Haven needle exchange participants: updated estimates from syringe tracking and testing data. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:175–176. [DOI] [PubMed] [Google Scholar]
- 32.Macalino GE, Vlahov D, Sanford-Colby S, et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. Am J Public Health. 2004;94:1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seage GR III, Holte SE, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol. 2001;153:619–627. [DOI] [PubMed] [Google Scholar]
- 34.Des Jarlais DC, Perlis T, Arasteh K, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95:1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collier K, Kotranski L, Semaan S, Lauby J, Halbert J, Feighan K. Correlates of HIV seropositivity and HIV testing among out-of-treatment drug users. Am J Drug Alcohol Abuse. 1998;24:377–393. [DOI] [PubMed] [Google Scholar]
- 36.Tobin KE, Tang AM, Gilbert SH, Latkin CA. Correlates of HIV antibody testing among a sample of injection drug users: the role of social and contextual factors. AIDS Behav. 2004;8:303–310. [DOI] [PubMed] [Google Scholar]
- 37.Vernon KA, Mulia N, Downing M, Knight K, Riess T. “I don’t know when it might pop up”: understanding repeat HIV testing and perceptions of HIV among drug users. J Subst Abuse. 2001;13:215–227. [DOI] [PubMed] [Google Scholar]
- 38.Riess TH, Kim C, Downing M. Motives for HIV testing among drug users: an analysis of gender differences. AIDS Educ Prev. 2001;13:509–523. [DOI] [PubMed] [Google Scholar]
- 39.Davis WR, Deren S, Beardsley M, Wenston J, Tortu S. Gender differences and other factors associated with HIV testing in a national sample of active drug injectors. AIDS Educ Prev. 1997;9:342–358. [PubMed] [Google Scholar]
- 40.Liebman J, Brooks D, Bonilla L, Kotranski L. Utilization of HIV antibody testing by out-of-treatment drug users. J Drug Issues. 1996;26:679–693. [Google Scholar]
- 41.Wurcel A, Zaman T, Zhen S, Stone D. Acceptance of HIV antibody testing among inpatients and outpatients at a public health hospital: a study of rapid versus standard testing. AIDS Patient Care STDS. 2005; 19:499–505. [DOI] [PubMed] [Google Scholar]
- 42.Spielberg F, Branson BM, Goldbaum GM, et al. Choosing HIV counseling and testing strategies for outreach settings: a randomized trial. J Acquir Immune Defic Syndr. 2005;38:348–355. [PubMed] [Google Scholar]
- 43.Anonymous. Supplemental testing for confirmation of reactive oral fluid rapid HIV antibody tests. MMWR [dispatch]. 2005;54:1287–1288. [Google Scholar]
- 44.Ekwueme DU, Pinkerton SD, Holtgrave DR, Branson BM. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25: 112–121. [DOI] [PubMed] [Google Scholar]
- 45.Thorpe LE, Bailey SL, Huo D, Monterroso ER, Ouellet LJ. Injection-related risk behaviors in young urban and suburban injection drug users in Chicago (1997–1999). J Acquir Immune Defic Syndr. 2001;27: 71–78. [DOI] [PubMed] [Google Scholar]
- 46.Des Jarlais DC, Perlis T, Arasteh K, et al. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. J Acquir Immune Defic Syndr. 2004;35: 158–166. [DOI] [PubMed] [Google Scholar]
- 47.Heimer R, Khoshnood K, Buchanan D, et al. Syringe exchange, community coverage, declining AIDS rates. Paper presented at: 2005 National HIV Prevention Conference; June 13, 2005; Atlanta, Ga.
- 48.Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11:F59–F65. [DOI] [PubMed] [Google Scholar]
- 49.Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–893. [DOI] [PubMed] [Google Scholar]
- 50.“HIV-infection” Information Bulletin No. 1 [in Russian]. Russian Federal AIDS Center; 1994. In: Gardner KJ. HIV Testing and the Law in Russia; 1996. Available at: http://www.openweb.ru/aesop/eng/hiv-hr/hiv.html. Accessed May 25, 2005.
- 51.Rhodes T, Sarang A, Bobrik A, Bobkov A, Platt L. HIV transmission and HIV prevention associated with injecting drug use in the Russian Federation. Int J Drug Policy. 2004;15:1–16. [Google Scholar]
- 52.National Institutes of Health Consensus Development Program. Interventions to prevent HIV risk behaviors. Bethesda, Md: National Institutes of Health; 1997. Report 104.
- 53.Holtgrave DR, Pinkerton SD, Jones TS, Lurie P, Vlahov D. Cost and cost-effectiveness of increasing access to sterile syringes and needles as an HIV prevention intervention in the United States. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S133–S138. [DOI] [PubMed] [Google Scholar]
- 54.Pollack HA, Heimer R. Impact and cost-effectiveness of methadone maintenance programs for HIV and hepatitis C prevention. In: Weissing L, ed. Impact and Costs of Hepatitis C in Injecting Drug Users in the European Union. Utrecht, Netherlands: European Monitoring Centre for Drugs and Drug Addiction; 2003.
- 55.Wood E, Montaner JS, Bangsberg DR, et al. Expanding access to HIV antiretroviral therapy among marginalized populations in the developed world. AIDS. 2003;17:2419–2427. [DOI] [PubMed] [Google Scholar]
- 56.Altice FL, Springer S, Buitrago M, Hunt DP, Friedland GH. Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. J Urban Health. 2003; 30:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Altice FL, Mezger JA, Hodges J, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38(suppl 5): S376–S387. [DOI] [PubMed] [Google Scholar]
- 58.Vlahov D, Galai N, Safaeian M, et al. Effectiveness of highly active antiretroviral therapy among injection drug users with late-stage human immunodefiniciency virus infection. Am J Epidemiol. 2005; 161:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C, Vlahov D, Galai N, et al. Mortality in HIV-seropositive versus seronegative persons in the era of highly active antiretroviral therapy: implications for when to initiate therapy. J Infect Dis. 2004;190: 1046–1054. [DOI] [PubMed] [Google Scholar]


