Abstract
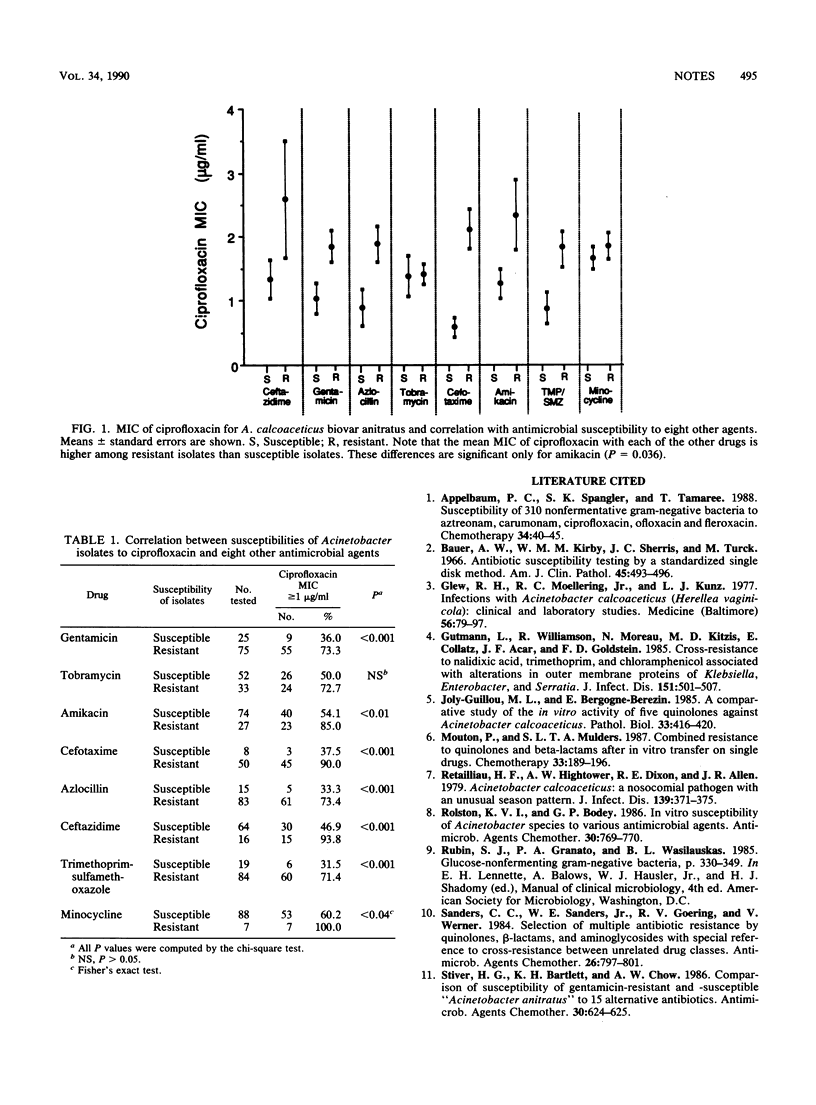
Using an agar dilution assay, for 66 of 104 (63.5%) clinical isolates of Acinetobacter calcoaceticus biovar anitratus, the MIC of ciprofloxacin was greater than or equal to 1.0 micrograms/ml. Cross resistance was demonstrable to ciprofloxacin and gentamicin (P less than 0.001), amikacin (P less than 0.01), cefotaxime (P less than 0.001), azlocillin (P less than 0.001), ceftazidime (P less than 0.001), trimethoprim-sulfamethoxazole (P less than 0.001), and minocycline (P less than 0.05). The mean MIC of ciprofloxacin for drug-susceptible isolates was consistently lower than that for resistant isolates; however, these differences were significant only for amikacin (P = 0.036).
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C., Spangler S. K., Tamarree T. Susceptibility of 310 nonfermentative gram-negative bacteria to aztreonam, carumonam, ciprofloxacin, ofloxacin and fleroxacin. Chemotherapy. 1988;34(1):40–45. doi: 10.1159/000238546. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Glew R. H., Moellering R. C., Jr, Kunz L. J. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): clinical and laboratory studies. Medicine (Baltimore) 1977 Mar;56(2):79–97. doi: 10.1097/00005792-197703000-00001. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Joly-Guillou M. L., Bergogne-Berezin E. Etude comparative in vitro de l'activité de cinq quinolones vis-à-vis d'Acinetobacter calcoaceticus. Pathol Biol (Paris) 1985 May;33(5):416–420. [PubMed] [Google Scholar]
- Mouton R. P., Mulders S. L. Combined resistance to quinolones and beta-lactams after in vitro transfer on single drugs. Chemotherapy. 1987;33(3):189–196. doi: 10.1159/000238494. [DOI] [PubMed] [Google Scholar]
- Retailliau H. F., Hightower A. W., Dixon R. E., Allen J. R. Acinetobacter calcoaceticus: a nosocomial pathogen with an unusual seasonal pattern. J Infect Dis. 1979 Mar;139(3):371–375. doi: 10.1093/infdis/139.3.371. [DOI] [PubMed] [Google Scholar]
- Rolston K. V., Bodey G. P. In vitro susceptibility of Acinetobacter species to various antimicrobial agents. Antimicrob Agents Chemother. 1986 Nov;30(5):769–770. doi: 10.1128/aac.30.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver H. G., Bartlett K. H., Chow A. W. Comparison of susceptibility of gentamicin-resistant and -susceptible "Acinetobacter anitratus" to 15 alternative antibiotics. Antimicrob Agents Chemother. 1986 Oct;30(4):624–625. doi: 10.1128/aac.30.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]


