Abstract
The present study examined perception of individual finger forces during multi-finger force production tasks. In an ipsilateral force matching paradigm, twelve healthy subjects were instructed to produce a reference force pre-determined at 30% MVC of involved fingers (varying from 1 to 4 fingers, visual feedback of total force, 5 s), and then to reproduce only the index or little finger portion of the total reference force (i.e., a portion of the sum, no visual feedback, 4 s) after a brief relaxation period (visual feedback of all finger forces, 3 s). The absolute force that individual fingers produced was approximately 30% of single-finger maximal force across different multi-finger reference force production tasks. During subsequent force matching, the index finger matching force was not significantly different from its own reference force, independent of the number of simultaneously activated fingers. The little finger, in contrast, produced significantly greater matching forces when three (middle, ring, and little) or four (index, middle, ring and little) fingers, but not two (ring and little) fingers, were simultaneously activated. The results suggest that index finger forces are more accurately estimated than little finger forces during multi-finger force production. The disparity in perception of individual finger forces is likely due to the ability of the central nervous system to partition and direct descending motor commands to the index finger.
Independent finger movements, though allowing superior manual dexterity, are not perfect in the human hand. It has been a common observation that force production with an explicitly instructed finger, i.e., a single-finger task, tends to result in force production in adjacent uninstructed fingers during submaximal and maximal voluntary contraction (MVC). This observation has been termed enslaving [7, 9, 15, 25]. Enslaving effects are more evident during ring and little finger tasks than during index finger tasks [24], suggesting more independence in force production by the index finger than by the ring and little fingers.
Perception of individual finger movements is a prerequisite for precise control of finger forces. Recently, Li and Leonard [14] employed a new ipsilateral matching paradigm to examine perception of finger force during single-finger tasks. For example, subjects were instructed to match the absolute magnitude of an index finger (reference) force at 40% MVC using the little (matching) finger of the same hand after a brief relaxation period. The little finger produced significantly smaller matching forces than the index finger reference forces. The matching errors (the difference between reference and matching forces), however, were significantly minimized when the sum of finger forces were compared during the reference and matching force production periods. The results suggested that both instructed and uninstructed finger forces were perceived within the central nervous system (CNS) during single-finger force production. Perception of individual finger movement, however, is altered by simultaneous activation of adjacent digits. Kilbreath and Gandevia [8] reported that perceived heaviness of a weight lifted by one digit progressively increased when the weight lifted by an adjacent digit increased, suggesting that finger force perception could be altered by simultaneous activation of adjacent fingers of the same hand, i.e., multi-finger force production.
Many skilled manipulative tasks are multi-finger tasks and individual finger forces continuously change during these tasks. Precise control of individual finger forces and moments of force is required, e.g., to provide pen stabilization during hand-writing [11] and grasp stability during grasping tasks [5, 18, 19]. In these circumstances, perception of individual finger forces becomes extremely important because it allows individual fingers to be integrated into a meaningful synergy for desired functions.
The present study aimed to examine perception of individual finger force during multi-finger force production tasks using an ipsilateral force matching paradigm. The index or little finger was instructed to reproduce its own force during multi-finger reference force production at 30% MVC. Given that finger forces are perceived differently [10] and the index finger is more independent in force production than other fingers [24], it was hypothesized that the index finger force would be more accurately reproduced than the little finger force during multi-finger reference force production.
Twelve healthy volunteers (1 male, 11 females; ranging from 23 to 52 years of age) took part in the experiments. All subjects were right-handed according to their preferential use of the right hand during writing and eating. The Institutional Review Board of the University of Montana approved the study and all procedures were consistent with The Helsinki Declaration. All subjects gave written informed consent prior to experiments.
During testing, subjects were seated on an adjustable chair. The two upper limbs were symmetrical with respect to the body midline with the upper arms at approximately 45° of abduction in the frontal plane and 45° of flexion in the sagittal plane, elbow joints at approximately 135° of flexion. The right forearm was stabilized at the proximal and distal sites by vertical aluminium bars. The distance between two bars that were placed in the medial and lateral sides of the forearm at each site was medio-laterally adjustable. Fingers were slightly curved (about 20º flexion of interphalangeal joints) such that finger forces were produced by pressing against the force sensors at fingertips isometrically while preserving the described configuration. The sensors were mounted inside a steel frame (140 mm x 90 mm each). The sensors were medio-laterally distributed 30 mm apart within the frame and adjustable in the forward-backward direction within the range of 60 mm. The steel frame with the sensors was placed between two horizontal aluminium bars that allowed horizontal adjustment of the frame. Such apparatus could accommodate individual subject's anatomy. This apparatus has been previously described in detail ([12]).
At the beginning of the experiment, subjects were asked to produce MVCs during single-finger tasks using the index (I), middle (M), ring (R) and little (L) fingers, respectively. The interval between two successive MVC attempts was approximately one minute. The highest peak value from three attempts of each task was considered as MVCi (i=I, M, R, L). The MVC of a multi-finger task was calculated as the sum of individual MVCs for the following tasks: IM, RL, IMR, MRL, and IMRL. The reference forces, equal to 30% of corresponding MVCs, were established for single- and multi-finger tasks. The reference force for each task was displayed to subjects on the computer screen by a thick red line.
Force matching tasks were then conducted. A matching task consisted of three sequential components: 1) matching to the pre-determined reference force (visual feedback of the total force, 5 s); 2) relaxation of all fingers (visual feedback of all finger forces to assure complete force relaxation of all fingers, 3 s); and 3) reproduction of the instructed finger force during multi-finger reference force tasks (no visual feedback, 4 s). For example, during IMRL/I tasks (Fig 1A), subjects were instructed to produce a reference force at the pre-determined level of 30% MVC of the sum of MVCi (i = I, M, R, L) using four fingers, to relax for 3 s, and then to reproduce the absolute magnitude of the index finger force (i.e., a portion of the sum) and sustain this force till the end of the trial. During the first 5 s of each trial, the subjects were explicitly instructed to match the red line on the screen, i.e., the predetermined reference force. The following matching conditions were tested: 1) index finger tasks: I/I, IM/I, IMR/I, IMRL/I; 2) little finger tasks: L/L, RL/L, MRL/L, IMRL/L. In these conditions, the index or little finger was instructed to reproduce its own force (bold letters).
Figure 1.
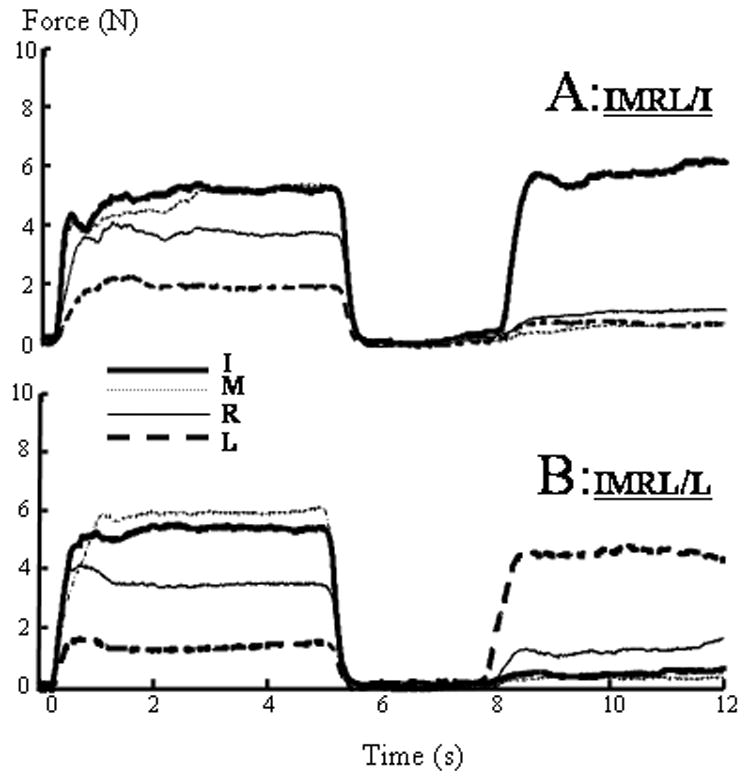
Typical force traces of IMRL/I and IMRL/L trials from the same subject. The subject matched the reference force of the index finger (A) but overestimated that of the little finger (B) during the same multi-finger (IMRL) force production. I: index finger; M: middle finger; R: ring finger; L: little finger.
Approximately 8 to 10 practice trials of randomly selected matching tasks (both index and little finger tasks) were allowed for each subject to familiarize themselves with the instructions. During practice trials, subjects received visual and verbal feedback in order to assure constant matching force effort during the last 4-s matching period. The interval between two consecutive trials was approximately 20 seconds. Each matching condition was conducted in a block of five trials. The order of matching conditions was randomized. Subjects received no visual or verbal feedback regarding force matching accuracy during the experiment.
Four unidirectional piezoelectric force sensors (208C02, PCB Piezotronics, Inc., Depew, NY) were used to measured individual finger forces. Analog output signals from the sensors were connected to separate signal conditioners (model 484B11, PCB Piezotronics). Force signals were sampled at 1000 Hz using a 16-bit analog-to-digital converter (PCI-6229, National Instruments, Austin, TX). The system involved ~1% error over the typical epoch of recording of a constant signal. The resolution of the system was 2.715 mN/bit. A PC desktop equipped with customized Lab VIEW software (National Instruments, Austin, Texas) was used for data acquisition and processing. A Matlab program was written for data analysis.
Similar to an earlier study [14], a 1.5-second period of force production for both reference and matching forces was selected to standardize data analysis when forces were most stable. The mean force value from 3.0 to 4.5 secondx of the reference force was measured as FREF, while the mean force value from 10.0 to 11.5 seconds of the matching force was calculated as FMATCH (i.e., 0.5 second prior to the end of force production).
Absolute forces (in Newtons) of the instructed index and little fingers were used to compare reference and matching forces. Force matching error was the difference between the reference force and the matching force. The absolute value of matching error (absolute error) has been viewed as a good index to assess matching accuracy because errors with different signs (under- or over-estimation) do not cancel each other out [6]. Absolute error was normalized to the instructed finger reference force. The normalized absolute error was defined as relative error. Parameters were averaged from five repeated trials for a given matching condition.
To compare reference and matching forces of the instructed finger, repeated measures ANOVAs were used for the index and little finger tasks separately, with factors NUMBER (4 levels; 1, 2, 3, 4 fingers involved in the reference force production), MATCH (2 levels; reference and matching). Separate one-way ANOVAs with the factor NUMBER were used to assess the effect of number of fingers involved in the task on absolute errors in the index and little finger tasks. To compare relative errors across fingers, a two-way ANOVA was performed with factors NUMBER and FINGER (2 levels; index and little). Multivariate ANOVAs (MANOVAs) were used to compare the pattern of force distribution among fingers (sharing) during IMRL tasks with a factor TASK (2 levels, index [IMRL/I] and little finger tasks [IMRL/L]). Rao’s R was used to detect the significance of the test. Sharing patterns were compared using three shares (e.g., I, M, R), because four individual shares did not constitute a set of independent variables (the sum is always 100%) (cf.[3]). Whenever necessary, post-hoc Tukey’s honest significant difference tests were utilized. The level of significance was set at p < 0.05.
The absolute reference force of the index finger or the little finger was not significantly changed across different multi-finger reference force productions. The index finger force was 29.9%, 31.9%, 28.6% and 31.0% of its MVC during I, IM, IMR, and IMRL reference force productions, respectively. The little finger force was 29.8%, 27.7%, 29.2%, and 28.3% of its MVC during L, RL, MRL, IMRL reference force productions, respectively. According to the MANOVA analysis, the pattern of finger force distribution among fingers, force sharing, during the reference force production (IMRL) was not significantly different between IMRL/I and IMRL/L tasks.
The index finger reference force appeared to be more accurately estimated during multi-finger tasks than the little finger reference force (Fig 1). Averaged across all subjects (Fig. 2), no statistically significant differences between the reference and matching forces of the index finger were found when the number of fingers increased. In contrast, the little finger matching force was significantly greater than the little finger reference force (F[1,11]=18.73, p<0.001), and increased with the increasing number of fingers (F[3,33]=6.37, p<0.002). ANOVAs indicated a significant interaction of FINGER × NUMBER (F[3,33]=9.61, p<0.001). Post-hoc tests indicated that the little finger matching force was significantly greater than the little reference force during MRL/L and IMRL/L tasks, but not during RL/L and L/L tasks.
Figure 2.
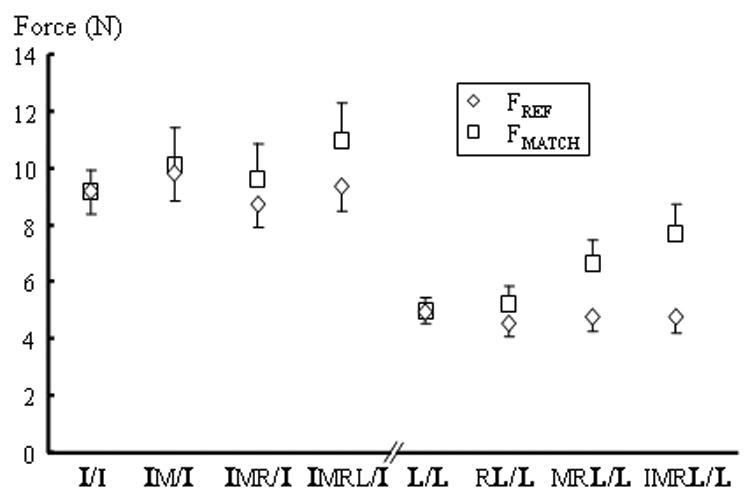
Absolute reference (FREF) and matching (FMATCH) forces for index and little finger tasks. In tasks with multi-finger reference force production (e.g. IM in IM/I tasks), forces from the finger (in bold letter) were measured as FREF. Forces were averaged across trials and subjects. Standard error bars are shown.
Absolute errors (Fig. 3A) showed a similar pattern of results as seen in the analysis of absolute forces. No statistical differences in absolute errors were found for the index finger tasks (I/I, IM/I, IMR/I, IMRL/I). Absolute errors for the little finger tasks increased with the increasing number of fingers during reference force production (F[3,33]=10.97, p<0.001). Post-hoc tests showed that absolute errors were significantly larger for IMRL/L tasks than for RL/L and L/L tasks, and that absolute errors for MRL/L tasks were significantly larger for L/L tasks (p<0.027). No differences in absolute errors between MRL/L and IMRL/L or between RL/L and L/L were found.
Figure 3.
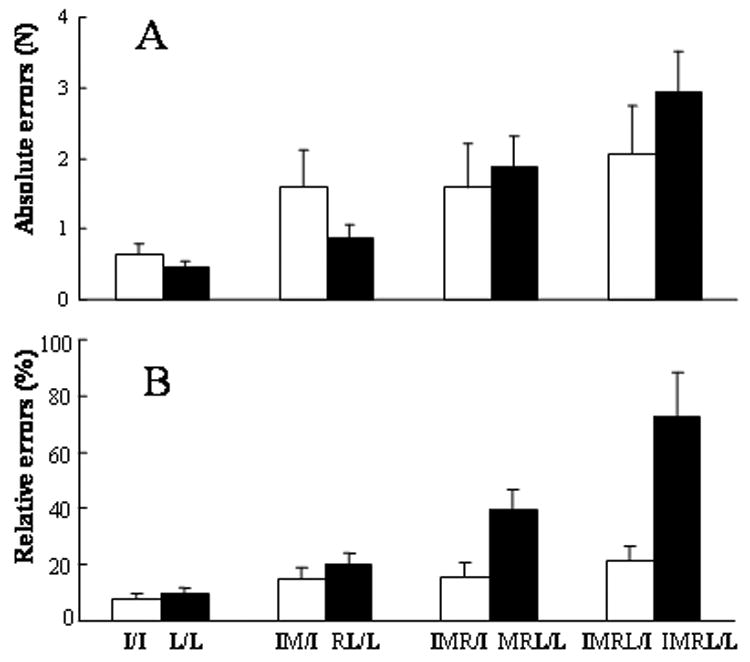
Absolute errors (A) and relative errors (B) for index and little finger tasks. Means and standard error bars are presented.
To compare matching errors across fingers, relative errors were used by normalizing absolute errors to the reference force of the instructed finger (Fig. 3B). Relative errors were significantly greater during little finger force matching (average, 35.6%) than during index finger force matching (average, 15.1%) (F[1,11]=8.89, p=0.012). There were also a main effect of NUMBER (F[3,33]=11.23, p<0.001) and a significant interaction of NUMBER × FINGER (F[3,33]=5.87, p=0.003). Post-hoc tests showed that relative errors were significantly greater in MRL/L tasks than in L/L tasks, and that relative errors in IMRL/L tasks were significantly larger than relative errors in all other matching conditions (p<0.005), among which relative errors were not statistically significant from each other.
It appears that the index finger reference force was more accurately estimated during multi-finger force production tasks than the little finger reference force. As the number of fingers in multi-finger force production increased, matching errors increased in little finger force matching, while the accuracy of force matching was not significantly changed in index finger force matching.
In the currently employed matching paradigm, the reference force is perceived within the CNS, and the matching force is then produced by retrieving information from memory. In an earlier study [14] using the same paradigm, the reference force produced by the index finger or the little finger could be accurately approximated by the matching force of the same finger (index-index or little-little matching) from 10% to 40% MVC of the instructed finger during single-finger matching tasks. When a reference force was instructed to be matched using a different finger (index-little or little-index matching), matching errors increased (particularly at 30% and 40%MVC), but were significantly minimized when the sum of instructed and uninstructed finger forces were compared. These findings suggested that both instructed and uninstructed finger forces were perceived during both reference and matching force production periods [14]. In the present study, the number of fingers involved in the reference force production was altered, while the matching force was produced solely by the instructed finger, i.e., single-finger force production. The memory of finger force was not likely to be distorted after a 3-s relaxation period (cf. [17]) and no difference in retrieving memory has been reported for the index or little finger. Furthermore, it is unlikely that changes in force generating capabilities [2] would contribute to present results. Force generating capability remained the same in matching tasks (index-index, or little-little matching). The results that matching errors increased with the number of simultaneously activated fingers during little finger tasks, but not during index finger tasks suggest that simultaneous activation of adjacent fingers could alter perception of little finger force, but not index finger force during multi-finger force production.
It’s well known that a single-finger movement can lead to widespread activation of the M1 hand area [20, 22]. The CNS has the ability with certain constraints [23] to selectively partition and direct descending motor commands to target muscles. In a recent TMS study [13], when asked to imagine flexion force production at the fingertips without actual execution (lack of peripheral inputs), subjects were able to distinguish one vs. four-finger imagined force production and selectively modulate the corticospinal excitability. This ability, however, is different among fingers. Within the distributed network of highly interconnected finger representations in the M1 hand area, the thumb and index have greater representations with relatively selective gradients, as compared to the ring and little fingers [21, 22]. Furthermore, the index finger demonstrates a high degree of neuromuscular compartmentalization among muscle compartments in the multitendoned FDP and FDS [1, 9]. For example, selective activation of middle finger component at about 25% MVC in FDP and about 50% MVC in FDS started to recruit neighboring index finger motor units. Recruitment thresholds for little finger motor units, by contrast, were low in both FDS and FDP during ring finger voluntary activation, at about only 5% MVC [1, 9].
Based on these findings, it could be reasonably inferred that each finger could produce force independently, when the level of voluntary activation of adjacent fingers is lower than the level for motor unit recruitment threshold. Conceivably, mediated by the central mechanism via the efferent copy, the ability to reproduce its own force by the index finger is not likely to be significantly disturbed when activation of adjacent fingers is around or below the recruitment threshold, resulting in no significant changes in matching errors during multi-finger reference force production as compared to single-finger force matching. The opposite, however, is expected for the little finger in this study. Furthermore, during reference force production involving the same fingers (IMRL), the significantly higher errors in IMRL/L tasks than in IMRL/I tasks suggests that the descending motor command is better partitioned and directed for the index finger than for the little finger. The proposed mechanism, however, needs to be further tested by examining matching behaviors at higher levels of reference force, especially higher than the motor unit recruitment threshold for the index finger.
The observed matching behaviors could be mediated through peripheral mechanisms [10, 14]. FDS exhibits ‘reflex partitioning’, i.e., each of its motoneuron sub-pools has a distinct pattern of cutaneomuscular reflexes. Cutaneomuscular reflexes of the index finger are facilitatory, leading to an increased perceived heaviness after index finger anaesthesia, while the corresponding effect is inhibitory in the little finger [10]. This mechanism is unlikely to explain the current results. The effect of cutaneomuscular reflexes remains the same for each finger (index and little) during reference and matching force production. If the central motor commands are modified by the inhibitory effect of cuteneomuscular reflexes in the little finger during multi-finger reference force production (e.g., IMRL), the little finger is expected to produce smaller force during subsequent force matching. Similarly, the index finger is expected to produce greater matching force, resulting in matching errors. In a recent study aiming to investigate the accuracy of the sense of tension [16], subjects were asked to estimate and reproduce the peak forces in the wrist extensors induced by transcranial magnetic stimulation (TMS) and magnetic stimulation of the radial nerve. The accuracy of force perception by this non-voluntary, non-reflex contraction with either cortical or peripheral stimulation was poor. This observation thus implies that the sense of tension is not likely to provide a reliable source of information for perception of muscle force, such as in tasks of multi-finger force production.
The present results of no significant change in matching errors in the index finger tasks are not consistent with a previous report [8], in which errors of perceived heaviness increased when the magnitude of concurrently lifted weight increased. The contrasting results may be related to different tasks. In the weight estimation study [8], the level of activation for the adjacent finger was managed by increasing the magnitude of the concurrently lifted weight. For example, the index finger lifted 200g while the ring concurrently lifted 300g. This procedure may alter the normal force sharing pattern between the index finger and the ring finger when these two fingers voluntarily produce forces. The level of index finger activation may increase when the magnitude of concurrently lifted weight increased, resulting in increased errors in perceived heaviness of the weight lifted by the index finger. In this study, the level of activation for the index finger remained relatively the same, about 30% MVC, irregardless of the number of fingers involved in the task.
The results suggest that the index finger force is more accurately estimated than the little finger force during multi-finger force production. The disparity in perception of individual finger force is likely due to the ability of the CNS to partition and direct descending motor commands to the index finger. This digit-specific finger force perception might contribute to the preferential use of the more independent fingers, e.g., index finger, during multi-finger tasks [4], such as grasping with the thumb and the index finger.
Acknowledgments
Valuable suggestions from Dr. Charles Leonard are highly appreciated. The author is also grateful to anonymous reviewers for their constructive suggestions. This study was supported in part by an NIH grant (1R15NS053442-01A1).
References
- 1.Butler TJ, Kilbreath SL, Gorman RB, Gandevia SC. Selective recruitment of single motor units in human flexor digitorum superficialis muscle during flexion of individual fingers. J Physiol. 2005;567:301–309. doi: 10.1113/jphysiol.2005.089201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson RG, Riek S, Shahbazpour N. Central and peripheral mediation of human force sensation following eccentric or concentric contractions. J Physiol. 2002;539:913–925. doi: 10.1113/jphysiol.2001.013385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danion F, Latash ML, Li ZM, Zatsiorsky VM. The effect of fatigue on multifinger co-ordination in force production tasks in humans. J Physiol. 2000;523 Pt 2:523–532. doi: 10.1111/j.1469-7793.2000.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edin BB, Westling G, Johansson RS. Independent control of human finger-tip forces at individual digits during precision lifting. J Physiol. 1992;450:547–564. doi: 10.1113/jphysiol.1992.sp019142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson RS, Backlin JL, Burstedt MK. Control of grasp stability during pronation and supination movements. Exp Brain Res. 1999;128:20–30. doi: 10.1007/s002210050813. [DOI] [PubMed] [Google Scholar]
- 6.Jones LA. Perceptual constancy and the perceived magnitude of muscle forces. Exp Brain Res. 2003;151:197–203. doi: 10.1007/s00221-003-1434-4. [DOI] [PubMed] [Google Scholar]
- 7.Kilbreath SL, Gandevia SC. Independent control of the digits: changes in perceived heaviness over a wide range of force. Exp Brain Res. 1992;91:539–542. doi: 10.1007/BF00227850. [DOI] [PubMed] [Google Scholar]
- 8.Kilbreath SL, Gandevia SC. Independent digit control: failure to partition perceived heaviness of weights lifted by digits of the human hand. J Physiol (Lond) 1991;442:585–599. doi: 10.1113/jphysiol.1991.sp018810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilbreath SL, Refshauge K, Gandevia SC. Differential control of the digits of the human hand: evidence from digital anaesthesia and weight matching. Exp Brain Res. 1997;117:507–511. doi: 10.1007/s002210050247. [DOI] [PubMed] [Google Scholar]
- 11.Latash ML, Danion F, Scholz JF, Zatsiorsky VM, Schoner G. Approaches to analysis of handwriting as a task of coordinating a redundant motor system. Hum Mov Sci. 2003;22:153–171. doi: 10.1016/s0167-9457(02)00157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Latash ML, Yue GH, Siemionow V, Sahgal V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clin Neurophysiol. 2003;114:1646–1655. doi: 10.1016/s1388-2457(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Latash ML, Zatsiorsky VM. Effects of motor imagery on finger force responses to transcranial magnetic stimulation. Cogn Brain Res. 2004;20:273–280. doi: 10.1016/j.cogbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Leonard CT. The effect of enslaving on perception of finger forces. Exp Brain Res. 2006;172:301–309. doi: 10.1007/s00221-005-0332-3. [DOI] [PubMed] [Google Scholar]
- 15.Li ZM, Latash ML, Newell KM, Zatsiorsky VM. Motor redundancy during maximal voluntary contraction in four-finger tasks. Exp Brain Res. 1998a;122:71–78. doi: 10.1007/s002210050492. [DOI] [PubMed] [Google Scholar]
- 16.Nicolas G, Marchand-Pauvert V, Lasserre V, Guihenneuc-Jovyaux C, Pierrot-Deseilligny E, Jami L. Perception of non-voluntary brief contractions in normal subjects and in a deafferented patient. Exp Brain Res. 2005;161:166–179. doi: 10.1007/s00221-004-2056-1. [DOI] [PubMed] [Google Scholar]
- 17.Nowak DA, Rosenkranz K, Hermsdorfer J, Rothwell J. Memory for fingertip forces: passive hand muscle vibration interferes with predictive grip force scaling. Exp Brain Res. 2004;156:444–450. doi: 10.1007/s00221-003-1801-1. [DOI] [PubMed] [Google Scholar]
- 18.Pataky TC, Latash ML, Zatsiorsky VM. Prehension synergies during nonvertical grasping, I: experimental observations. Biol Cybern. 2004;91:148–158. doi: 10.1007/s00422-004-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pataky TC, Latash ML, Zatsiorsky VM. Prehension synergies during nonvertical grasping, II: Modeling and optimization. Biol Cybern. 2004;91:231–242. doi: 10.1007/s00422-004-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford: 1993. [Google Scholar]
- 21.Schieber MH. Constraints on Somatotopic Organization in the Primary Motor Cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- 22.Schieber MH. Somatotopic gradients in the distributed organization of the human primary motor cortex hand area: evidence from small infarcts. Exp Brain Res. 1999;128:139–148. doi: 10.1007/s002210050829. [DOI] [PubMed] [Google Scholar]
- 23.Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- 24.Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biol Cybern. 1998;79:139–150. doi: 10.1007/s004220050466. [DOI] [PubMed] [Google Scholar]
- 25.Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]


