Abstract
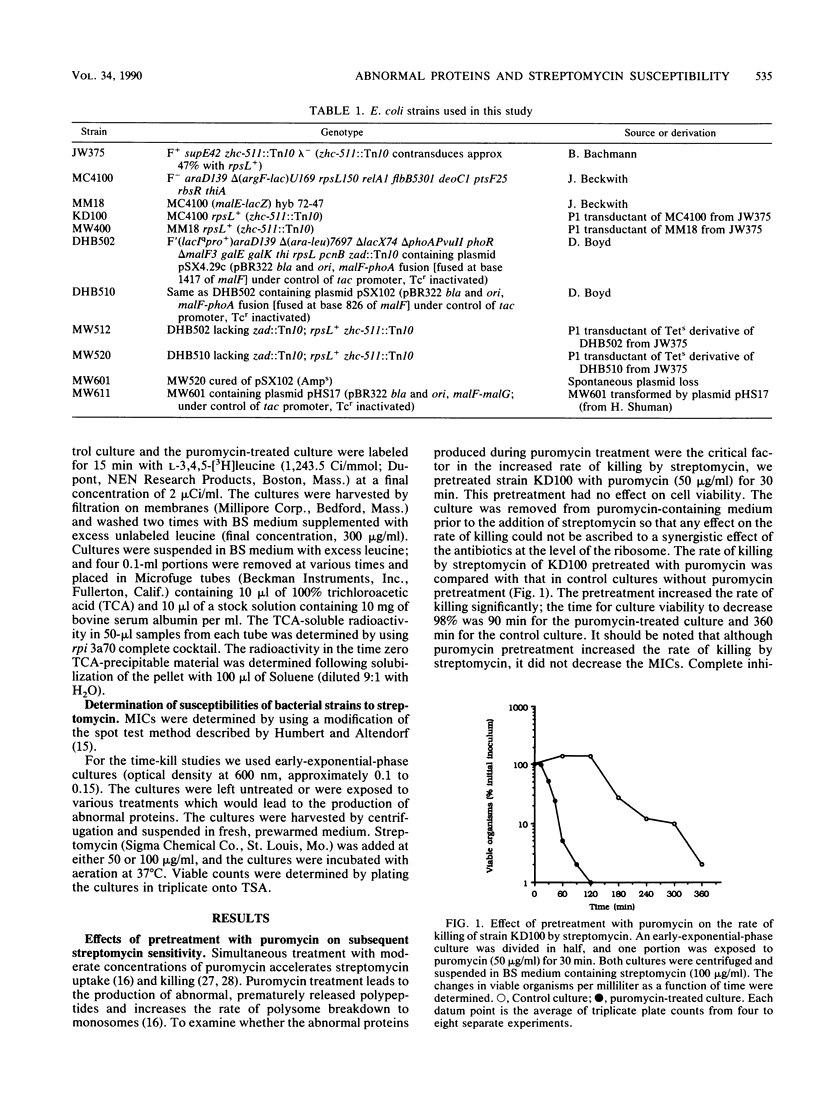
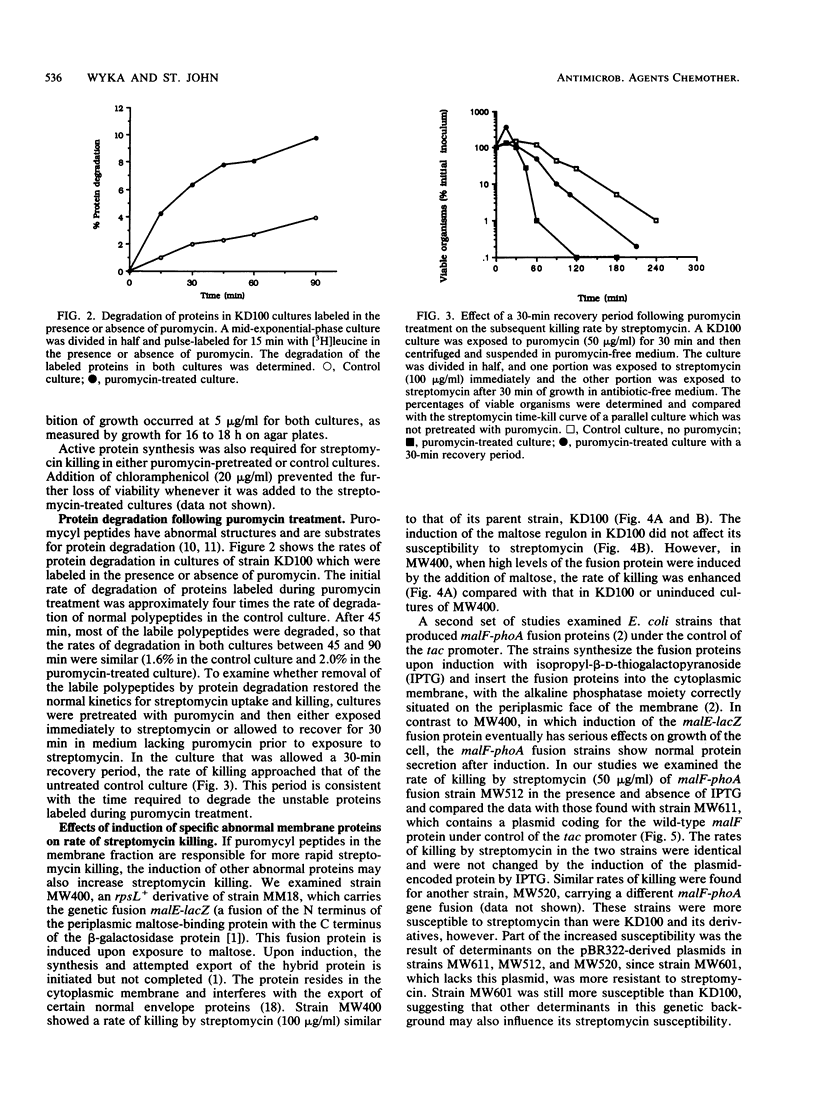
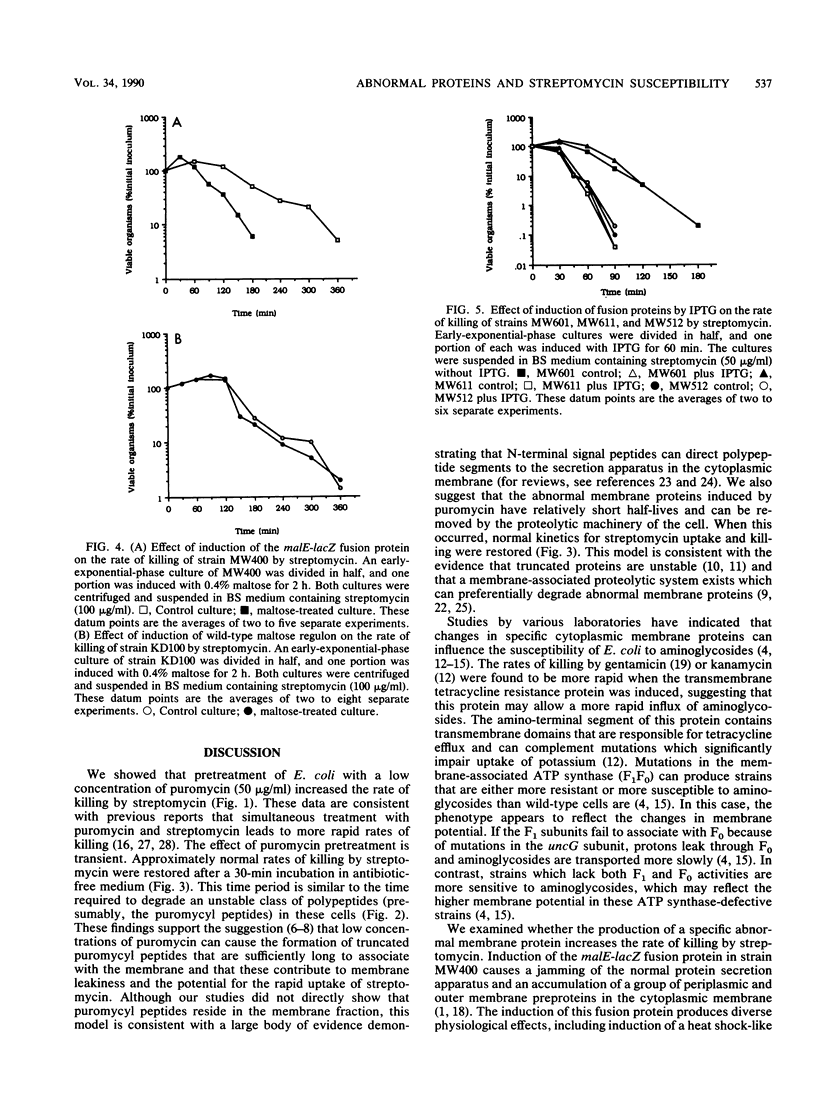
The role of abnormal membrane proteins in modulating the rate of killing by streptomycin was investigated. Davis et al. (B.D. Davis, L. Chen, and P.T. Tai, Proc. Natl. Acad. Sci. USA 83:6164-6168, 1986) have proposed that misread membrane proteins created by the action of streptomycin on translating ribosomes cause the formation of nonspecific membrane channels which allow increased uptake of the antibiotic and contribute to its bactericidal action. Pretreatment of Escherichia coli with a low concentration of puromycin enhanced the rate of killing by streptomycin. The effect of the pretreatment with puromycin was transient, since approximately normal rates of killing by streptomycin were restored after 30 min of incubation in antibiotic-free medium. This time period correlates with the time required to degrade labile polypeptides in puromycin-treated cells. The induction of a specific abnormal malE-lacZ fusion protein, which is capable of disrupting the normal membrane protein secretion process, also increased the rate of killing by streptomycin. Induction of malF-phoA fusion proteins, which have no significant effects on membrane integrity, did not alter susceptibility to streptomycin. These observations suggest that certain abnormal membrane proteins can contribute to the bactericidal action of streptomycin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassford P. J., Jr, Silhavy T. J., Beckwith J. R. Use of gene fusion to study secretion of maltose-binding protein into Escherichia coli periplasm. J Bacteriol. 1979 Jul;139(1):19–31. doi: 10.1128/jb.139.1.19-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Manoil C., Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Chen L. L., Tai P. C. Misread protein creates membrane channels: an essential step in the bactericidal action of aminoglycosides. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6164–6168. doi: 10.1073/pnas.83.16.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987 Sep;51(3):341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D. Non-specific membrane permeability and aminoglycoside action. J Antimicrob Chemother. 1989 Jul;24(1):77–78. doi: 10.1093/jac/24.1.77. [DOI] [PubMed] [Google Scholar]
- Gentz R., Kuys Y., Zwieb C., Taatjes D., Taatjes H., Bannwarth W., Stueber D., Ibrahimi I. Association of degradation and secretion of three chimeric polypeptides in Escherichia coli. J Bacteriol. 1988 May;170(5):2212–2220. doi: 10.1128/jb.170.5.2212-2220.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Griffith J. K., Kogoma T., Corvo D. L., Anderson W. L., Kazim A. L. An N-terminal domain of the tetracycline resistance protein increases susceptibility to aminoglycosides and complements potassium uptake defects in Escherichia coli. J Bacteriol. 1988 Feb;170(2):598–604. doi: 10.1128/jb.170.2.598-604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action-with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981 Dec;8(6):429–445. doi: 10.1093/jac/8.6.429. [DOI] [PubMed] [Google Scholar]
- Humbert R., Altendorf K. Defective gamma subunit of ATP synthase (F1F0) from Escherichia coli leads to resistance to aminoglycoside antibiotics. J Bacteriol. 1989 Mar;171(3):1435–1444. doi: 10.1128/jb.171.3.1435-1444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz C., Braun C. B., Rosano C. L. Role of ribosome recycling in uptake of dihydrostreptomycin by sensitive and resistant Escherichia coli. Biochim Biophys Acta. 1981 Jan 29;652(1):168–176. doi: 10.1016/0005-2787(81)90220-3. [DOI] [PubMed] [Google Scholar]
- Ito K., Akiyama Y., Yura T., Shiba K. Diverse effects of the MalE-LacZ hybrid protein on Escherichia coli cell physiology. J Bacteriol. 1986 Jul;167(1):201–204. doi: 10.1128/jb.167.1.201-204.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Merlin T. L., Corvo D. L., Gill J. H., Griffith J. K. Enhanced gentamicin killing of Escherichia coli by tet gene expression. Antimicrob Agents Chemother. 1989 Feb;33(2):230–232. doi: 10.1128/aac.33.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W. The enigma of streptomycin transport. J Antimicrob Chemother. 1989 May;23(5):673–676. doi: 10.1093/jac/23.5.673. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Young S. N. Respiration-dependent uptake of dihydrostreptomycin by Escherichia coli. Its irreversible nature and lack of evidence for a uniport process. Biochem J. 1985 Jun 1;228(2):505–512. doi: 10.1042/bj2280505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S. M., St John A. C. Characterization of a membrane-associated serine protease in Escherichia coli. J Bacteriol. 1987 Apr;169(4):1474–1479. doi: 10.1128/jb.169.4.1474-1479.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Werner P. K., Müller M. Insertion of proteins into bacterial membranes: mechanism, characteristics, and comparisons with the eucaryotic process. Microbiol Rev. 1989 Sep;53(3):333–366. doi: 10.1128/mr.53.3.333-366.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. W., Mueller J. P., Miller P. F., Arrow A. S. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987 Dec;51(4):439–457. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE J. R., WHITE H. L. STREPTOMYCINOID ANTIBIOTICS: SYNERGISM BY PUROMYCIN. Science. 1964 Nov 6;146(3645):772–774. doi: 10.1126/science.146.3645.772. [DOI] [PubMed] [Google Scholar]
- YAMAKI H., TANAKA N. EFFECTS OF PROTEIN SYNTHESIS INHIBITORS ON THE LETHAL ACTION OF KANAMYCIN AND STREPTOMYCIN. J Antibiot (Tokyo) 1963 Nov;16:222–226. [PubMed] [Google Scholar]


